Full-Mouth Root Coverage: A Combined Approach Using Connective Tissue and an Acellular Dermal Matrix
Michael Sonick, DMD; Debby Hwang, DMD
Gingival recession compromises esthetics, comfort (via hypersensitivity) and, in cases of severe attachment loss, possibly tooth longevity. Numerous etiologies explain the presence of recession: periodontal diseases, thin gingiva and bone, orthodontic movement, subgingival restorations, abrasion, erosion, periodontal therapy, snuff use, foreign body impaction, periand intraoral piercings, high frenum, or muscle attachment. There appear to be as many ways to resolve it.1 Indeed, the majority of research in periodontal plastic surgery concerns root coverage, especially as society increasingly focuses on appearance enhancement.
Introduced more than 20 years ago by Nelson and modified by Langer, Langer, and Calagna for recession correction, the subepithelial connective tissue graft (CTG) remains the gold standard among treatment modalities.2,3 It appears to give the greatest frequency of complete root coverage and the most consistent, stable, and natural-looking results.1,4 Palatal connective tissue is most often the graft source; the chief drawbacks to CTG use then are morbidity, bleeding, and a finite amount of donor tissue.5 Compared with other sorts of periodontal surgery, autogenous free grafting procedures associate with more severe postoperative complications, including pain, infection, and swelling, presumably because of the presence of a second surgical site (donor).6,7
The extent of coverage with a CTG depends on palatal anatomy. A wide, thick palate is ideal. Measurements on Caucasian adults suggest that the greatest mean palatal thickness (roughly 4 mm) occurs 12 mm from the papilla between the first and second maxillary molars as well as 12 mm from the free gingival margin of the first premolar.8 The thickness increases with the distance away from the teeth.
That said, the key consideration in graft harvest is the location of the greater palatine foramen, which must not be encroached. The greater palatine foramen lies roughly 3 mm to 4 mm anterior to the posterior border of the hard palate, at the junction of the alveolar and palatine processes, which varies with palatal vault depth.9 Palatal vault depth is the shortest distance between the midline of the hard palate and the cementoenamel junction (CEJ) of the first maxillary molar. In those with shallow vaults, the mean distance of the greater palatine foramen from the first molar CEJ is 7 mm.10 People with high, U-shaped vaults have a mean distance of 17 mm. Notably, subjects with average vault depths exhibit foramina 12 mm apical to the first molar CEJ, where the tissue, as mentioned previously, is thickest and most amenable for grafting. Thus, before harvesting, the surgeon must determine the exact position of the foramen because it may compromise the amount of tissue collected.
Acellular dermal matrix (ADM) allografts have been in use in medicine for skin replacement, lip augmentation, and other reconstructive surgery. Their fabrication entails processing of human dermis to remove cells and render HIV and hepatitis C levels nondetectable. No reported cases of disease transmission exist. An ADM contains no immunogenic cellular components but retains intact, nonimmunogenic vasculature, collagen, ground substance, and elastic fibers, which promote host-cell establishment.11,12 Like dermis, the material has two sides: the basement membrane side, which is smooth and not blood absorbent; and the connective tissue side, which is rough and blood absorbent.11,12 With such properties, an ADM may serve as a membrane for guided tissue regeneration.13 Healing of an ADM, like that of an autogenous graft, is primarily by repair (scar) instead of regeneration.14,15
An ADM has the same indications as an autogenous CTG, including ridge augmentation, augmentation of keratinized mucosa, vestibular deepening, and tattoo masking. Arguably, its most common use is for root coverage, especially for widespread recession. As a cadaver product, an ADM has a virtually unlimited source of donor tissue and precludes the need for a second harvest site—both characteristics that connective tissue grafting lacks.
Does recession correction with ADM measure up to the outcomes generated with CTG? A meta-analysis noted comparable mean root coverage and color match between ADM, CTG, and coronally positioned flaps.16 The range of coverage reported for ADM spanned 50% to 99%. Very few studies, however, met the inclusion criteria (randomized controlled trial), and most of the included investigations lasted only 6 months.
In contrast, several studies document the lasting efficacy of root coverage with connective tissue.17,18 ADM does not fare as well, probably because of its relatively recent application in dentistry and subsequent dearth of prolonged trials. One long-term investigation reveals that root coverage by ADM may not be stable over time, with decreases from 93% mean root coverage at 4 weeks to 66% after 4 years.19 One study found that ADM may shrink as much as 70% over a period of 6 months.20 On the other hand, another study showed that ADM exhibited creeping attachment or coronal displacement of tissue after 12 months, leading to a 1-mm gain in root coverage.21
To be a beneficial and cost-effective treatment, root coverage using ADM must equal but not necessarily exceed the results garnered by autogenous tissue because ADM's advantages over CTG are significant. The following scenario exemplifies this point.
Patients often have restricted time for dental visits; this mandates highly efficient care, which is especially tricky to manage in complex cases. One-time-only treatment appointments save time, boost productivity, and minimize discomfort. Often performed under conscious sedation, this approach to therapy avoids multiple surgeries and recovery periods and reduces time away from work, thus expediting treatment completion.
A patient with generalized gingival recession is a prime candidate for the above full-mouth method. The limiting factor, however, becomes the amount of donor tissue required. There is a set volume of autogenous tissue available based on palatal anatomy.8-10 A dilemma arises when little patient mucosa exists but broad coverage is required. The traditional resolution to that predicament involves extracting as much autogenous mucosa as possible (ie, bilateral palatal harvest), grafting as many sites as possible, and repeating the treatment when donor-site healing completes. After the first laborious procedure, the patient may be reluctant to pursue a follow-up surgery.
The solution presented here may be more practical and less traumatic. It is possible to obtain the maximum amount of autogenous connective tissue present and supplement any required additional mucosa with allograft, namely ADM. Use of allograft thus facilitates full-mouth coverage, negating the need for a second appointment. The following three cases of maxillary and mandibular recession illustrate this approach.
Case 1: Maxillary CTG Using Envelope Approach; Mandibular ADM Using Envelope Approach
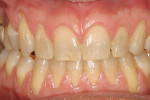
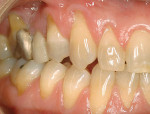
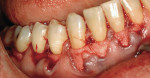
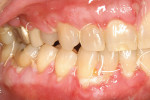
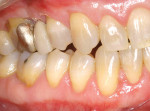
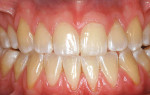
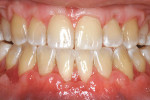
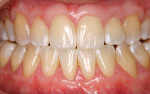
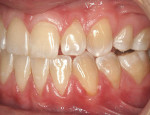
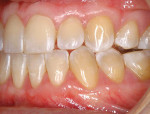
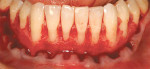
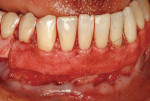
A nonsmoking, medically and periodontally healthy 44-year-old man presented with progressive gingival recession and hypersensitivity of both maxillary and mandibular teeth. On examination, Miller class I recession was detected from teeth Nos. 3 through 7 and Nos. 24 through 30, with depths ranging from 1 mm to 5 mm15 (Figure 1 and Figure 2). The patient also exhibited similar recession contralaterally on teeth Nos. 8 through 14 and Nos. 19 through 25. Because of the extent of the recession, two autogenous subepithelial CTGs were chosen to treat the maxillary right teeth, while an ADM (AlloDerm®, BioHorizons Inc, Birmingham, AL) was selected to correct the mandibular left dentition. It was decided to treat the opposite side at a future visit.
Recipient Sites
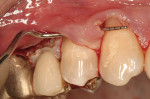
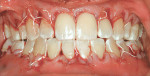
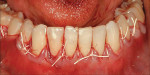
After intravenous sedation and local anesthetic induction, a buccal sulcular incision was made from tooth No. 2 to the distal of tooth No. 6. The papilla between teeth Nos. 6 and 7 was maintained and a modified tunnel procedure was performed (Figure 3). Full-thickness dissection occurred, without violation of the papillary tissue. Split-thickness dissection was executed at the base of the flap to allow for coronal repositioning. No vertical releasing incisions were made. A modified tunnel approach, as described by Allen,16 was used for the mandibular site (from tooth No. 24 to No. 30), with elevation of alternate papilla to facilitate flap manipulation (Figure 4). In all areas, the exposed root surfaces were scaled and root planed with a 7/8 Gracey curet and Neumeyer bur. Tetracycline solution was applied for 2 minutes, after which the root surfaces were irrigated with sterile water.
Donor Sites
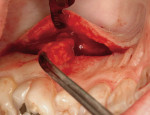
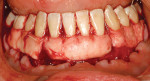
Following the protocol described by Langer and Langer,2 subepithelial CTGs were harvested bilaterally from the palate (distal aspect of the canine to mesial aspect of the second molar), avoiding the greater palatine foramina (Figure 5). The palatal incisions were closed primarily via interrupted suturing with 4-0 expanded polytetrafluoroethylene (ePTFE). The two CTGs were trimmed with a No. 15 scalpel blade to comply with defect morphology.
The 1 cm x 4 cm ADM graft was processed according to the manufacturer’s directions. The ADM was hydrated in sterile saline for 15 minutes until the backing could be removed easily and then placed in a second sterile saline bath for 10 minutes. The material was trimmed to fit the mandibular defects.
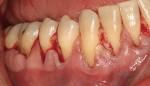
Graft Placement positioned over teeth Nos. 3 through 7 and secured via sling and periosteal suturing with 5-0 plain gut (Figure 6). The flap was advanced to cover the CTGs and sutured in place with 4-0 ePTFE. One intact segment of ADM was positioned over teeth Nos. 24 through 30 and secured via sling and periosteal suturing with 5-0 plain gut and 6-0 polyglactin 910 (Figure 7). The flap was advanced to cover the ADM and sutured in place with 4-0 ePTFE and 6-0 polyglactin 910 suture (Figure 8).
Postoperative Instructions
The patient was prescribed ibuprofen 600 mg every 4 hours and hydrocodone 7.5 mg/acetaminophen 750 mg as required for analgesia, and doxycycline 100 mg once daily for 10 days as antibiotic. The patient was instructed not to use a toothbrush but instead to rinse with 0.12% chlorhexidine or warm saline twice daily. The ePTFE sutures were removed 7 days postsurgery.
Clinical Results
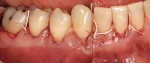
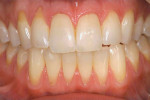
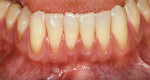
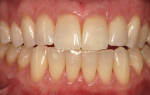
At 1 week after surgery, healing was within normal limits in the maxilla (Figure 9). Mild sloughing, however, occurred in the mandible. The sloughing was mostly on the marginal gingiva where the allograft was not covered completely. At 9 months, healing was complete (Figure 10).
Case 2: Maxillary CTG Using Envelope Approach; Mandibular ADM Using Envelope Approach
A nonsmoking, medically and periodontally healthy 18-year-old woman presented with progressive gingival recession, hypersensitivity, and esthetic compromise of her maxillary and mandibular teeth. On examination, Miller class I recession was detected from teeth Nos. 5 through 12, and Miller class II recession from teeth Nos. 19 through 30, with depths ranging from 1 mm to 3 mm22 (Figure 11). Because of the extent of the recession, an autogenous subepithelial CTG was chosen to treat the maxillary teeth, while an ADM (AlloDerm) was selected to correct the mandibular dentition.
Recipient Sites
After intravenous sedation and local anesthetic induction, buccal sulcular incisions were made from teeth Nos. 3 through 14 as well as from teeth Nos. 18 through 30. Full-thickness envelope flaps were elevated.23 No vertical releasing incisions were made. In all areas, the exposed root surfaces were scaled and root planed with a 7/8 Gracey curet and Neumeyer bur. Tetracycline solution was applied for 2 minutes, after which the root surfaces were irrigated with sterile water.
Donor Sites
The subepithelial CTG was harvested using the same protocol as described for Case 1. Then, one 1 cm x 2 cm ADM graft was processed according to manufacturer’s directions.
Graft Placement
Two CTG were positioned over teeth Nos. 5 through 12 and secured via sling and periosteal suturing with 5-0 plain gut. The flap was advanced to cover the CTG and sutured in place with 4-0 ePTFE (Figure 12). Three segments of ADM were positioned over teeth Nos. 18 through 30 and secured via sling and periosteal suturing with 5-0 plain gut (Figure 13). The flap was advanced to cover the ADMs and sutured in place with 4-0 ePTFE (Figure 13).
Postoperative Instructions
The patient received the same postoperative prescriptions and instructions as the patient in Case 1. However, for this case, the ePTFE sutures were removed 14 days postsurgery (Figure 14).
Clinical Results
Healing was within normal limits. The patient went to college and was only seen every 6 months for recall. Excellent root coverage in the maxilla and mandible was evident at the 18-month follow-up (Figure 15). Root coverage appeared to have been maintained both in the maxillary autogenous grafted areas as well as the mandibular allograft sites (Figure 16a, Figure 16b, Figure 16c, and Figure 16d). Tissue thickness also was improved.
Case 3: Maxillary CTG Using Envelope Approach; Mandibular ADM Using Envelope Approach
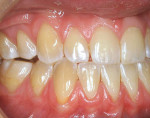
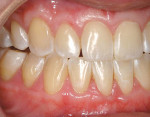
A nonsmoking, medically and periodontally healthy 19-year-old woman presented with esthetic compromise and hypersensitivity of her maxillary and mandibular teeth. On examination, Miller class I and II recession was detected from teeth Nos. 6 through 11 and from teeth Nos. 20 through 29, with depths ranging from 1 mm to 4 mm15 (Figure 17 and Figure 18 ). Because of the extent of the recession, an autogenous subepithelial CTG was chosen to treat the maxillary teeth, while an ADM (AlloDerm) was selected to correct the mandibular dentition.
Recipient Sites
After intravenous sedation and local anesthetic induction, buccal sulcular incisions were made from teeth Nos. 6 through 11 as well as from teeth Nos. 19 through 30 (Figure 19). Split-thickness envelope flaps were elevated.23 No vertical releasing incisions were made. In all areas, the exposed root surfaces were scaled and root planed with a 7/8 Gracey curet and Neumeyer bur. Tetracycline solution was applied for 4 minutes, after which the root surfaces were irrigated with sterile water.
Donor Sites
The subepithelial CTG was harvested using the same protocol as described for Case 1. Then, one 1 cm x 4 cm ADM graft was processed according to manufacturer's directions.
Graft Placement
The CTG was positioned over teeth Nos. 6 through 11 and secured via sling and periosteal suturing with 5-0 plain gut. The flap was advanced to cover the CTG and sutured in place with 4-0 ePTFE. Two segments of ADM were positioned over teeth Nos. 20 through 29 and secured via sling and periosteal suturing with 5-0 plain gut (Figure 20). The flap was advanced to cover the ADMs and sutured in place with 4-0 ePTFE suture (Figure 21).
Postoperative Instructions
The patient received the same postoperative prescriptions and instructions as the patient in Case 2. The ePTFE sutures were removed 7 days postsurgery.
Clinical Results
Healing was within normal limits. At the 3-year follow-up, complete root coverage and adequate tissue thickness in both mandible and maxilla had been maintained (Figure 22).
Conclusion
A viable adjunct to or substitute for autograft in root coverage, allograft exhibits two superior features: easy availability and reduced morbidity. Preparation of only one surgical site hastens procedure completion and diminishes swelling and pain afterward. The use of allograft condenses into one appointment treatment that conventionally requires several visits. This convenience is of utmost importance in patients with generalized gingival recession but scheduling constraints or dental anxiety. Recession correction in these full-mouth cases may consist of CTG and ADM (as described previously) or ADM monotherapy.
There is no strong consensus on which defects favor one material or another. A reflection of vascularity, flap thickness at the recipient site may directly influence the mean root coverage attained by coronally positioned flaps.24 Thicknesses of at least 1 mm associate with better results.25 Although not mandatory, primary closure may be performed for ADM grafting; if a coronally positioned flap is planned to accomplish this, then the surgeon may want to select a recipient site with thicker tissue to sustain survival of not only the flap but also the ADM graft.
Alternately, the flap may be designed to preserve patent blood flow. A revision of the envelope flap, which omits vertical incisions, the tunneling technique avoids violation of the papilla, thus maximizing vascularization of the donor graft.23,26 This vascularization is particularly helpful in regions with thin mucosa, and it is feasible that tunnel preparation could allow for graft survival in otherwise inhospitable sites.
It is clear that both autografts and allografts have discrete advantages in root coverage. The clinician must choose use based on patient needs and site morphology. A renewable resource, ADM functions as a convenient donor tissue. Initial reports show promise, but its long-term stability remains to be seen. As clinical familiarity and scientific evidence with it accumulate, ADM may turn out to be invaluable for periodontal plastic surgery.
Disclosure
Dr. Sonick has received grant/research support from Brasseler USA.
References
1. Sonick MK, Hwang D. Periodontal plastic surgery I: root coverage. Inside Dentistry. 2007;3(5):76-80.
2. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56(12): 715-720.
3. Langer B, Calagna L. The subepithelial connective tissue graft. J Prosthet Dent. 1980;44(4):363-367.
4. Clauser C, Nieri M, Franceschi D, Pagliaro U, Pini-Prato G. Evidence-based mucogingival therapy. Part 2: Ordinary and individual patient data meta-analyses of surgical treatment of recession using complete root coverage as the outcome variable. J Periodontol. 2003;74(5):741-756.
5. Sato N. Periodontal surgery: a clinical atlas. Vol 1: Quintessence; 2000.
6. Curtis JW, Jr., McLain JB, Hutchinson RA. The incidence and severity of complications and pain following periodontal surgery. J Periodontol. 1985;56(10):597-601.
7. Powell CA, Mealey BL, Deas DE, McDonnell HT, Moritz AJ. Post-surgical infections: prevalence associated with various periodontal surgical procedures. J Periodontol. 2005;76(3):329-333.
8. Studer SP, Allen EP, Rees TC, Kouba A. The thickness of masticatory mucosa in the human hard palate and tuberosity as potential donor sites for ridge augmentation procedures. J Periodontol. 1997; 68(2):145-151.
9. Clarke MA, Bueltmann KW. Anatomical considerations in periodontal surgery. J Periodontol. 1971;42(10):610-625.
10. Reiser GM. Root coverage utilizing the subepithelial connective tissue graft. Dent Econ. 1995;85(4):90-91.
11. Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Periodontol. 1998; 69(11): 1305-1311.
12. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21(4):243-248.
13. Novaes AB, Jr., Souza SL. Acellular dermal matrix graft as a membrane for guided bone regeneration: a case report. Implant Dent. 2001;10(3): 192-196.
14. Richardson CR, Maynard JG. Acellular dermal graft: a human histologic case report. Int J Periodontics Restorative Dent. 2002;22(1):21-29.
15. Wei PC, Laurell L, Lingen MW, Geivelis M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 2002;73(3):257-265.
16. Gapski R, Parks CA, Wang HL. Acellular dermal matrix for mucogingival surgery: a meta-analysis. J Periodontol. 2005; 76(11):1814-1822.
17. Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession. A systematic review. Ann Periodontol. 2003;8(1):303-320.
18. Roccuzzo M, Bunino M, Needleman I, Sanz M. Periodontal plastic surgery for treatment of localized gingival recessions: a systematic review. J Clin Periodontol. 2002; 29 Suppl 3:178-194; discussion 195-176.
19. Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75(5):734-743.
20. Wei PC, Laurell L, Geivelis M, Lingen MW, Maddalozzo D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. A clinical study. J Periodontol. 2000;71(8):1297-1305.
21. Haeri A, Parsell D. Creeping attachment: autogenous graft vs dermal matrix allograft. Compend Contin Educ Dent. 2000;21(9):725-729; quiz 730.
22. Miller PD, Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8-13.
23. Allen AL. Use of the supraperiosteal envelope in soft tissue grafting for root coverage. I. Rationale and technique. Int J Periodontics Restorative Dent. 1994;14(3): 216-227.
24. Baldi C, Pini-Prato G, Pagliaro U, et al. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J Periodontol. 1999;70(9): 1077-1084.
25. Hwang D, Wang HL. Flap thickness as a predictor of root coverage: a systematic review. J Periodontol. 2006;77(10):1625-1634.
26. Zabalegui I, Sicilia A, Cambra J, Gil J, Sanz M. Treatment of multiple adjacent gingival recessions with the tunnel subepithelial connective tissue graft: a clinical report. Int J Periodontics Restorative Dent. 1999; 19(2):199-206.
About the Authors
Michael Sonick, DMD
Director
Sonick Seminars
Fairfield, Connecticut
Debby Hwang, DMD
Private Practice
Fairfield, Connecticut