The Expansion of Millable Materials
New additions to the market increase patient-care options
By Gregg Helvey, DDS, MAGD
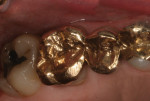
The only constant in dental material technology is gold (Figure 1). This restorative material has the longest history of use in dentistry, going as far back as to when the pyramids were being constructed. All other restorative materials have either become obsolete or have been modified to enhance their physical properties. The first ceramics used in dentistry (feldspathic glass) have an irregular pattern of spacing and an amorphous structure, exhibiting low opacity and flexural strength, as well as high translucency. These ceramics are usually fused to a stronger core or substructure.1 From there, different filler particles are added to a glassy matrix to regulate optical effects such as opalescence, fluorescence, and shade, and to increase strength.2 Fillers can be mechanically added to the glass or grown inside the glass from a specific heat treatment called creaming, which produces a growth of crystals derived from glass itself. Leucite was the first crystalline mineral used as a filler.1 Depending on the classification of the ceramic, different fillers have been used to create specific ceramic physical properties. Examples include nepheline, albite, aluminia, magnesium aluminate spinel, or a mixture of alumina and zirconia.2 Germanium is a more recent addition to ceramic compositions.
To briefly summarize, the glassy matrix provides esthetics while the filler provides the strength. Therefore, the greater the volume of the glassy matrix, the higher the esthetic quality of a restoration will be, and the greater the volume of filler, the greater the strength of the restoration.3
The “Lithium” Family of Ceramic Systems
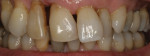
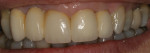
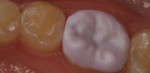
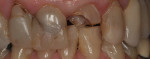
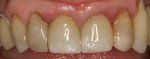
There are several all-ceramic systems that can be used to fabricate lithium based single and multi-unit restorations. Developed in response to a high demand for all-ceramic fixed partial dentures (FPDs), lithium disilicate was first introduced to the dental market in 1998 as IPS Empress® 2 (Ivoclar Vivadent, www.ivoclarvivadent.com). Initially, IPS Empress 2 was used mainly as a single-core or FPD framework to which a veneering ceramic was applied (Figure 2 and Figure 3).
Lithium disilicate (IPS e.max®), is composed of quartz, lithium dioxide, phosphor oxide, alumina, potassium oxide, and other components.4 This type of glass ceramic is available in two forms; a pressable version used in the laboratory (Figure 4) or a machinable form used in CAD/CAD milling (Figure 5 and Figure 6). Lithium disilicate CAD blocks come partially crystallized. In this state, the lithium metasilicate crystals provide sufficient strength for milling and try-in purposes. The manufacturing process uses a glass technology that provides a material void of any defects and an even distribution of color pigments. The grain sizes of lithium metasilicate crystals range from 0.2 μm to 1 μm, rendering this material a flexural strength of 130 MPa. Once milled, the pre-sintered ceramic is then crystallized. During this process there is a controlled growth of the grain size (0.5 μm to 5 μm). This transformation ultimately results in a glass ceramic made up of prismatic lithium disilicate dispersed in a glassy matrix. This alteration increases the flexural strength of the restoration to 360 MPa,5,6,7 an increase of 170%. A random orientation of small interlocking plate-like crystals makes up the lithium-disilicate restoration (Figure 7 and Figure 8).
A newer member of the “lithium” family is lithium silicate ceramic (Obsidian™, Glidewell Laboratories, www.glidewelldental.com). In addition to the basic components of silicon dioxide, aluminum dioxide, potassium oxide, and lithium oxide, this material also contains 7.6% germanium dioxide in the final composition. The addition of germanium dioxide provides a number of distinctive qualities to this ceramic system, including an increase in its optical properties, castability, thermal expansion, and refractive index. Germanium dioxide also increases the final density, resulting in a higher flexural strength since lithium silicate, not lithium disilicate, is the main constituent of the crystalline phase after full crystallization.5 This lithium silicate can be used for full-contour crowns, 3-unit anterior bridges, veneers, inlays, and onlays. According to the manufacturer, it has an average flexural strength of 373 MPa. These ceramic restorations can be inserted using conventional cementation or adhesively bonded.
The newest member of the “lithium” family is zirconia-reinforced lithium disilicate. Zirconium dioxide was introduced in dentistry in the early 2000s as a substructure for single crowns and fixed partial dentures, which subsequently were layered with veneering porcelain. Commonly referred to as “zirconia,” zirconium dioxide is now used as a full contour monolithic single crown and as multiple unit monolithic fixed partial dentures. Zirconia has also been incorporated in lithium silicate ceramic and referred to as zirconia reinforced lithium silicate or ZLS.
The new machinable glass ceramic is marketed by two companies under the name of Vita Suprinity® (Vident, www.vident.com) and Celtra™ Duo (DENTSPLY Prosthetics, prosthetics.dentsply.com). ZLS combines zirconia (10% by weight), which has—according to different internal studies conducted by the two manufacturers—an increase in its physical properties when compared to either milled or pressed lithium disilicate. In ZLS materials, the zirconium dioxide is in a highly dispersed form that prevents it from crystallizing and maintains a higher translucency than all-zirconia restorations. ZLS ceramic blocks are produced using glass technology (melting, cooling, uniform nucleation, and growth of crystals8) and poured into steel molds. According to the manufacturer, ZLS offers a homogeneous, fine crystalline structure with an average crystal size of 0.5 µm compared to the needle-shaped crystals with an average size of 1.5 µm found in the lithium disilicate ceramic.9,10 The addition of zirconia to the lithium silicate increases the flexural strength to approximately 420 MPa (16% increase). The manufacturer has demonstrated the difference in the edge stability between the lithium disilicate and the zirconia reinforced lithium silicate by milling a wedge-shaped 30° test specimen of each material using Sirona’s MC XL milling machine. They found, after milling, that the thickness of a stable edge for the ZLS ceramic measured as low as 163 µm as compared to the lithium disilicate at 275 µm. This allows the zirconia reinforce lithium silicate to achieve margins with a thickness of approximately 0.16 mm. They also found that the milling time (both normal and fast mode) for ZLS was 1-3 minutes less than the lithium disilicate. This greater edge stability also eliminates the use of an auxiliary firing paste during the crystallization process, whereas the lithium disilicate can distort under its own weight as the temperature increases and approaches a softening level during the crystallization process.11
There will be two forms of ZLS ceramic offered on the dental market—a fully crystallized version, which does not require the post-milling firing cycle, and a pre-crystallized version (Figure 6). The difference between the two versions (besides the required post-milling heat treatment) is that the fully crystallized form has a flexural strength of approximately 200 MPa, which is significantly lower than the pre-crystallized form. Both manufacturers plan to provide a pressable version of this new ceramic in the near future.
Millable Metals
Titanium has been milled in the laboratory for years and has a substantial track record. Beyond titanium, Chrome/Cobalt (CoCr) metal is another millable metal material used for single copings and multi-unit fixed partial denture frameworks. Milling dense metals like these often requires expensive and complex milling equipment. Amann Girrbach recently introduced a cost-effective manufacturing process for chrome/cobalt frameworks that can be completed by most in-house CAD/CAM milling machines. What makes this possible is that the chrome/cobalt/molybdenum alloy is milled in a pre-sintered state, similar to milling pre-sintered zirconia, and then sintered in a specific furnace developed for this material. Metal will oxidize in the presence of oxygen, and therefore argon gas replaces oxygen in these sintering furnaces. Under an argon shielding atmosphere, a preset temperature program tailored for this alloy reduces the milled unit to its pre-calculated finished contour and size.12 Simplifying this process allows the dental laboratory to provide a time-efficient and cost-effective CoCr framework.
Sirona’s inCoris CC ( www.sirona.com) is also a sintering metal based on a CoCr alloy. The process is similar to fabricating zirconia in that an oversized monolithic restoration or substructure is milled and then compressed to the proper size through sintering in a furnace in the presence of an argon atmosphere. After the oversized restoration is wet-milled by the inLab MC XL milling unit (Sirona), it can be sintered in 10 minutes using the high-speed inFire HTC (Sirona) furnace in the presence of argon gas.
Zirkonzahn also offers a chromium-cobalt alloy, Sintermetall ( www.zirkonzahn.com), which can be sintered under vacuum. This eliminates the need to purchase an additional sintering furnace. Both zirconia and the chrome-cobalt alloys can be sintered using the same Zirkonofen 700 Vakuum (Zirkonzahn) furnace.
DENTSPLY will also be offering a pre-sintered non-precious millable metal called Crypton that is similar to the previously mentioned millable chrome-cobalt blocks. It is milled 10% larger than the final restoration, then sintered in an oxygen-free argon atmosphere furnace (Multimat2Sinter, DENTSPLY), shrinking exactly to its absolute dimension. It will be available in 4 different block sizes, the smallest of which can accommodate the fabrication of 1 to 2 crowns, and the largest of which can fabricate more than 20 units.
Since a large number of non-precious alloy restorations are still used today, the biggest advantage in using a millable chrome/cobalt/molybdenum alloys is the time saving factor. The difference in quality and time to produce a homogenous, distortion-free, stress-free framework through digitally designing, milling, and sintering compared to the preparation of the model, waxing, investing, casting, and deflasking is easy to see.
Millable Plastics
Polymethyl methacrylate (PMMA) is a tooth-colored or clear acrylic material that has various uses in a dental laboratory that uses a CAD/CAM unit. It is a synthetic polymer of methyl methacrylate and was first introduced in 1928 under the trademark Plexiglas.13 The tooth-colored version can be used for provisional restorations, while the clear version can be used as a template for pressing ceramics to zirconia frames or as a test mill in difficult cases. Additionally, PMMA does not contain bisphenol-A (BPA), a chemical found in polycarbonate. The use of BPA has been restricted in some countries because of potential health hazards.
The Future of Millable Materials
The number of new millable dental materials continues to expand, and with this expansion, technicians now have alternative means of fabricating restorations. Additionally, the digital aspect of dental technology is gaining a greater presence in the laboratory, and will encourage the development of even newer materials that will offer users increased accuracy and shorter production times.
References
1. Helvey GA. A history of dental ceramics. Compend Contin Educ Dent. 2010;31(4):1-3.
2. Kelly RJ. Dental ceramics: what is this stuff anyway? J Am Dent Assoc. 2008;139(suppl 4):4S-7S.
3. Helvey GA. Classification of Dental Ceramics: An understanding of dental ceramic classifications enables the clinician to provide the optimum in strength and esthetics. Inside Dentistry. 2013;9(4):62-80.
4. Fasbinder DJ, Dennison JB, Heys D, Neiva G. A clinical evaluation if chairside lithium disilicate CAD/CAM crowns. A two-year report. J Am Dent Assoc. 2010;141(suppl 2):10S-14S.
5. Castillo R, inventor; James R. Glidewell Dental Ceramics Inc., assignee. Lithium silicate glass ceramic and method for fabrication of dental appliances. United States patent US7892995. February 22, 2011.
6. Helvey GA. Chairside CAD/CAM. Lithium-disilicate restoration for anterior teeth made simple. Inside Dentistry. 2009;5(10):58-67.
7. Tysowsky G. The science behind lithium disilicate: Today’s surprisingly versatile, esthetic and durable metal-free alternative. Oral Health Journal. 2009;March:93–97.
8. El-Meliegy and R. van Noort. Glasses and Glass Ceramics for Medical Applications.New York, NY: Springer; 2012:209-218.
9. IPS e.max® lithium disilicate: The Future of All-Ceramic Dentistry.Amherst, NY: Ivoclar Vivadent; 2009:1-15.
10. Fasbinder DJ. Chairside CAD/CAM: An overview of restorative material options. Inside Dentistry. 2012;8(5):58-65.
11. Völkel T. Accuracy of fit of all-ceramic restorations. Report: Research and Development Ivoclar Vivadent AG. 2006;16:23-26.
12. Noack DF. CoCr-Revolution. Dental Dialogue. 2012;13:2-5.
13. Plexiglas Triumphs. Rohm and Haas. 2012. Accessed January 30, 2014. https://archive.is/6oWT.
About the Author
Gregg Helvey, DDS, MAGDis an Adjunct Associate Professor at Virginia Commonwealth University School of Dentistry in Richmond, VAand has a private practice in Middleburg, VA.