CAD/CAM Milled Titanium Bar Restorations
A hot technology just keeps gaining momentum
The application of CAD/CAM milled titanium bars as a restorative solution for treating the edentulous as well as the partially edentulous arch is a rapidly growing sector of implant dentistry, as evidenced by the ever-increasing number of manufacturers that are expanding into this technology arena.
The emergence of this relatively new restorative solution offers great opportunity and excitement for dental laboratories and technicians. However, it is the author’s opinion that dental technicians have quickly reached a crossroads in this particular arena, and as an industry, should proceed cautiously with regard to the components and fabrication protocols that are selected to provide these highly complex and often challenging prostheses to our clinical customers and, ultimately, our patients.
It must be noted that the patient has not only paid a handsome fee for the elevated level of treatment, but most often, has also endured a very long process of clinical diagnosis, possibly extractions and regenerative procedures (bone grafting), surgical implant placement, provisionalization, and time to allow for proper osseointegration. Patient expectations for a highly successful final prosthesis—not only on the day of delivery but for weeks, months, and many years after—are greatly justified. Laboratories that choose to fabricate these devices must understand that there is a great deal of responsibility to ensure that those expectations are met.
Milling Center and Service Options
CAD/CAM milled titanium bars are readily obtainable from well known major implant companies that have produced bars for some time now, such as NobelProcera™, (Nobel Biocare, www.nobelbiocare.com), BellaTek® Bars (BIOMET 3i, www.biomet3i.com), Straumann ( www.straumann.us), and Dentsply Compartis® ISUS (prosthetics.dentsply.com).
There are also specialty milling companies like Cagenix (www.cagenix.com) or Preat Corporation (www.preat.com), and even a laboratory that provides bar restorations to other laboratories and dentists (www.glidewelldental.com), as well as third-party facilitators such as Dale Dental (www.daledental.com), that can assist laboratories in obtaining milled-bar solutions.
There are also numerous other smaller and lesser-known sources that have recently entered the market and rumors that several other implant companies plan to come on line with bar production this year.
There are even companies marketing smaller, more economical five-axis milling machines with bar-milling capabilities for laboratories, but their efficiency compared to the larger, more complex equipment used by the industrial milling centers is a debate for another time.
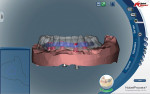
It is not the author’s intention to compare bar-milling companies or their products; however, it must be pointed out that there is quite a degree of variance in product, service, design style, and pricing among them. One of the main factors of consideration for discerning technologists and laboratories might be that some systems allow the laboratory to completely control the bar design aspect in-house while others design it for you and only allow for review of provided virtual design screenshots (Figure 1) with bar design modification based on feedback.
Bar design is critical and should never be oversimplified with a “connect the dots” approach. Although companies can quickly train computer-savvy representatives to adequately operate the design software, it requires a designer who is experienced and understands intraoral engineering and biomechanical concepts such as vertical and lateral load forces, their effects on the bar, and its relation to the denture teeth and base acrylic.
Bar Type Design Considerations
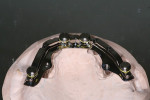
The most widely prescribed screw-retained hybrid or “fixed-detachable” bar (Figure 2) must have an adequate width on top with rounded corners. The bar must be properly positioned to support the anterior teeth and occlusal table of the posteriors, so that when tremendous vertical forces are applied, a cantilevered fracture fulcrum is not created.
The design must also account for minimal dimensional considerations to avoid failure due to outright breakage. Additionally, it must also resist flexure, especially in the mandible, as that too is a crucial element with regard to the long-term bonding of the teeth and base acrylic components to the bar. Proper finish line strategies are crucial as well as respecting denture tooth and acrylic thickness requirements so that they are not compromised due to improper bar placement.
In the case of an attached overdenture bar (Figure 3)—most commonly in the maxilla—the buccal and lingual walls must be tapered, usually at 4 degrees, to facilitate an intimate fit of the overdenture to help stabilize the prosthesis and transfer both vertical and horizontal forces directly to the bar, relegating the attachments to be only retentive elements as intended.
If unknown parties are designing the bar for your laboratory, it is highly recommended that the technologist and the clinician carefully examine all aspects of the design to ensure that it meets the desired and necessary requirements.
Ancillary Products and Services
It is important to recognize that milled-bar restorations are the final prostheses and often represent the back half of a larger picture that involves other products and services related to that same patient and treatment plan. With the widespread acceptance and use of Nobel Biocare’s All-on-4™ Immediate Load treatment plan concept (now offered by other systems as well), laboratory-processed, immediate-load provisionals, as well as the chairside “conversion” assistance service associated with that process, are very much in demand and command respectable fees. Laboratories that provide these services must possess a deep knowledge and understanding of the technology and realize the level of responsibility that is required, including possible legal ramifications.
There is also a considerable demand for laboratory-processed diagnostic devices and manual drilling guides (Figure 4) for conventional surgical implant placement, as well as CBCT scanning devices to assist with digitally planned and guided surgeries. These are required to ensure the strategic, prosthetic-driven placement of implants and proper bone reduction to allow for an adequate defect with regard to vertical space so that the final restoration will not be dimensionally compromised or cause a hygienic challenge due to a concave ridge-lap intaglio surface.
One of the most important and potentially time-consuming factors for laboratories operating in this arena to consider is that what you are providing is not just a product, but also a service. The fact is that this technology has been slow to be incorporated into the curriculum at many dental schools. Consequently, most general practitioners attempting their first or second cases require a great deal of assistance with the step-by-step procedures, such as open-tray, two-stage verified (splinted) impression techniques, and the “reverse engineering” principles of building the device on a screw-retained resin bar first to diagnose and satisfy all the esthetic and functional concerns before a bar style is committed, designed, and milled.
The importance of the laboratory’s ability to assist the clinician with identifying the necessary parameters and rationale to select the proper type and style of restoration as well as to identify any potential problems that might be encountered cannot be overstated.
The unfortunate truth is that many dental professionals, both clinical and technical, do not fully comprehend some of the basic principles of multiple implant solutions for the edentulous arch. These include:
• Understanding the difference between an implant-assisted prosthesis (direct attached and non-splinted; or splinted, but with inadequate A-P spread, using residual ridge/soft-tissue support), versus an implant-supported prosthesis (proper A-P spread between implants that are splinted; all load forces transferred directly to implants and bone, with no residual ridge/ soft-tissue support).
• Necessary vertical space requirements to avoid compromised prosthesis construction more prone to failure
• Tilted implants and divergence issues and how they relate to the long-axis screw-access shafts in fixed detachables, as well as proper use of angle correction abutments
• When the criteria dictates that an attached overdenture is indicated in the maxilla
• A-P spread and acceptable cantilever formulas
• Problematic implant systems for this technology due to interface issues and/or lack of proper prosthetic components, such as guide pins/chimney screws for setting/waxing, and processing fixed detachables.
These factors must be considered, discussed, understood, and agreed upon before a successful prosthesis can be designed, fabricated, and delivered to the patient. As a result, the process can be very time-consuming.
Parts Management
Parts management is an often overlooked and underestimated portion of the implant bar process. A laboratory that commits to restoring any and all implant systems must possess an organized database of prosthetic components and a comprehensive plan to either stock or otherwise readily obtain the many different necessary components, such as analogs, temporary abutments/cylinders, laboratory screws, guide pins, attachments, etc. Some clinicians even rely on the laboratory to provide typically clinical components such as impression copings and abutments. It is important to remember that all components for multiple implant restorative processes require non-engaging components.
It can be a very expensive proposition to stock parts for all the systems, platform sizes, and variations. It can be a daunting task to select and custom-order all necessary components in a timely fashion. Shipping charges to expedite shipment of custom-ordered parts can add up quickly. It is recommended that as soon as a case receives the green light to be fabricated, the entire process be reviewed and all parts that will be required be ordered and checked early on.
Where Are We Going From Here?
There are other similar yet distinctly different technologies available today for treating the edentulous arch (zirconia and alloy frameworks with ceramics, monolithic devices, etc.). With the rapid pace that materials science and CAD advancement are moving, it is difficult to predict how long titanium-bar restorations using denture teeth and PMMA base material will be relevant. However, their foreseeable future is looking bright due to their versatility, comparatively economical cost, the high esthetic quality of premium denture teeth, ease, and cost effectiveness to replace a failed tooth, or even completely retread when the teeth and subsequent vertical dimension of occlusion wears down. Some clinicians might argue that certain opposing dentition situations favor their wear characteristic.
It is interesting that even though we are now using modern technology such as CAD/CAM and improved components such as double cross-linked PMMA and nano-hybrid composite denture teeth such as BlueLine® DCL and SR Phonares® II (Ivoclar Vivadent, www.ivoclarvivadent.com), as well as injected base acrylic like the Ivocap® and IvoBase® system (Ivoclar Vivadent), we are still fabricating and placing devices that are very similar to the devices that Professor P.I. Brannemark first placed in Sweden in the mid-1960s (denture teeth processed to a bar with PMMA).
There is plenty of room for continued innovation in terms of bar design and elements, including friction fit concepts similar to those obtained in the past using spark erosion. Surely CAD/CAM mirror reflection, controlled taper, meso-structures cannot be too far away. Further development and use of the unique “fixed-removable” (as opposed to the fixed-detachable) prosthesis such as the Marius Bridge, should be expected as well (www.mariusbridge.com).
Conclusion
Laboratories should work together in harmony with our clinical partners to analyze, innovate, and improve our ability to communicate and properly engineer these prostheses to meet the rigid demands they are subjected to intraorally. This entire arena presents an opportunity for a more prestigious and rewarding position for the technician within the treatment flow process due to a more comprehensive contribution. As a profession, we should seize this opportunity instead of finding ways to quicken and cheapen the product in the name of competition and market share. Excessive compromise will certainly lead to highly undesirable consequences. Conversely, the competition should be one of technical design and engineering excellence.
To sign up for Tom Wade’s Master Level Workshops in Sarasota and Toronto, visit: dentalaegis.com/go/idt349
About the Author
Thomas Wade, CDT is the owner of New Horizons Dental Laboratory in Broomfield, Colorado.