Finishing Zirconia Chairside
What dental technicians need to tell their dentist clients about the effects of surface grinding.
By Gregg A. Helvey, DDS
The 1950s and 1960s have often been called the Golden Age of Dentistry—as patients flocked to dental offices to address oral health problems they had long neglected since the end of World War II. With such a wide variety of all-ceramic restorative materials available today, one could refer to the present as the Ceramic Age of Dentistry.
No material has had as dramatic a rise in popularity as zirconia. In the last 10 years, there has been a rapid development in the use of zirconia in medical and dental technology. Because of its improved strength, biocompatibility, and enhanced esthetics, zirconia is included in the term bioceramics.1 Zirconia’s primary use in dentistry was and still is as a substructure for single crowns and frameworks for fixed partial dentures. Material science and CAD/CAM technology have now propelled the use of zirconia in monolithic single-unit crowns and multiple-unit fixed partial dentures. Even though zirconia is considered a ceramic, technically, it is a metal oxide and has different properties than the other true ceramic materials used today.
Zirconia Properties
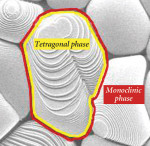
Depending on temperature and pressure formation, zirconia can exist in three forms—cubic, tetragonal, and monoclinic.2 Cubic, which has a straight prism and straight-sided form, is stable at temperatures above 2370°C and exhibits moderate mechanical properties. Tetragonal, which has a straight prism and rectangular-sided form, is stable between 1170°C and 2370°C and possesses improved mechanical properties. Monoclinic, which has a deformed prism with a parallelepiped form (prism with six faces), is stable at room temperatures up to 1170°C and has reduced mechanical properties.1-2 The tetragonal form is used in dentistry and medicine.
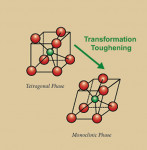
Zirconia possesses a unique characteristic called transformation toughening, which is the material’s unique ability to stop the growth of cracks. Tensile stresses generated by an imminent crack induce a change from the tetragonal configuration to a monoclinic configuration and a localized volume increase of 3% to 5% (Figure 1 and Figure 2). This volume increase results in a change of tensile stresses to compressive stresses generated around the tip of the crack (Figure 3 ). The compressive forces counter the external tensile forces and stop the crack from advancing.3-4 This characteristic accounts for the material’s low susceptibility to stress fatigue and high flexural strength of 900 MPa to 1200 MPa.5-6
To control phase transformations and stabilize zirconia at room temperature, different metal oxides such as yittria (Y2 O3) or ceria (CeO2), are added to the crystal structure.7 The addition of stabilizing oxides yields multiphase materials, called partially stabilized zirconia.8 Technically, if yittria is added for stabilization, then it is referred to as Yittria-stabilized Tetragonal Polycrystals (Y-TZP).2
Effect of Grinding on Zirconia
Grinding can increase the strength of Y-TZP zirconia.9 Micro cracks are produced by grinding, which results in the development of compressive strains from the transformation-related volume increase several microns below the surface.10 Micro-crack propagation is prevented by the surface compressive strains, resulting in an increase of the zirconia’s flexural strength.2 But before technicians begin to advise their doctors to have their way with grinding zirconia, certain criteria are essential. The severity (depth) of the grinding and the rise in the locally developed temperature will have an effect on the volume percentage of transformed zirconia (tetragonal phase to monoclinic phase).11-15 Using a 25-µ diamond bur increases the zirconia’s strength, whereas coarse grinding results in decreased strength. Grinding with a water coolant can promote the tetragonal-monoclinic transformation, which increases the surface compressive strength.11-12,16
Aging of Y-TZP zirconia
The aging of Y-TZP zirconia is a low-temperature degradation phenomenon. It is characterized by a progressive, spontaneous transformation of the tetragonal phase into the monoclinic phase (T-M), which results in diminished mechanical properties.17 Sato et al18 proposed that a slow T-M transformation occurs when Y-TZP is in contact with water, vapor, body fluid, or steam sterilization, which leads to surface damage. The reaction of water with zirconia at the tip causes a formation of zirconia hydroxide, which accelerates crack growth of pre-existing flaws and promotes the T-M phase transition.1 The most critical temperature range for the development of aging is 200°C to 300°C.19 Grinding zirconia without a water coolant can produce a white light or hot spot measuring up to 1500°C, which can induce a T-M phase change.
The literature shows conflicting studies concerning surface treatment of zirconia ceramics. For example, Guazzato et al20 suggest that the flexural strength of Y-TZP ceramics is increased by sandblasting and wet grinding, provided that the material is not followed with heat treatment. The heat relieves the compressive forces by reversing the monoclinic phase, which improves the flexural strength. On the other hand, Wang et al21 state that heat has no influence on the flexural strength whether it is performed before or after particle abrasion. This procedure is in need of further study. After evaluating the influence of surface grinding and sandblasting, Karakoca and Yilmaz22 found that the biaxial flexural strength of materials decreased after grinding and increased after sandblasting.
Guazzato et al20 also state that fine polishing may remove the layer of compressive stresses and therefore lower the mean flexural strength. Does one give up strength for a smoother surface to the opposing dentition?
Giordano23 suggests that any procedure that affects the surface of zirconia can cause a varying degree of phase change. He notes that there is an increase in the compressive stress, which increases the strength when zirconia surface is ground with a 125-µ grit diamond wheel. The strength is a result of the phase transformation. Subsequent heating will relieve the compressive stress, causing a reversal of the transformation; in fact, the strength will be reduced 30% from the original surface. This is because the damaged surface is still present. If a 50-µ and a 5-µ wheel are used to polish, then the damaged surface is removed and the original strength is returned. Giordano’s group also found that grinding, without polishing or heat-treating before porcelain application, increases the chances of the veneering porcelain cracking. Sandblasting also affects the surface, but does not create the same level of damage that grinding does.
Finishing and Polishing Zirconia
Finishing with diamond burs involves the indenting and scratching of the ceramic surface with sharp diamond particles, which can lead to subsurface damage.24 The diamond’s particle size influences the induced damage.25 Severe edge chipping or flaking occurs on feldspathic porcelain with both coarse and fine diamonds, but is absent with ultrafine diamond burs. Y-TZP zirconia is free of edge chipping for all grit sizes of diamond burs.25 A microscopic examination of the surface after finishing by a dental handpiece and diamond bur reveals a combination of ductile and brittle-type chip formation.25-27 The removal mechanism in the feldspathic porcelain is dominated by brittle fracture, while the removal process in Y-TZP zirconia is primarily achieved by a ductile mode.28
There are a number of finishing and polishing systems commercially available that are specific for zirconia-based crowns. These systems contain a series of diamond burs of various shapes and diamond-impregnated silicone cups and points. There are two classes of diamond burs—the common, less-expensive bonded diamond bur and the sintered diamond bur. They come in fine, medium, and coarse grit. The sintered diamond bur is made from a slurry of diamond particles and a binding material that is hot-pressed onto a bur shank. As a sintered diamond wears, the diamond chips are worn or abraded from the matrix, and new subsurface diamond particles are exposed to continue the cutting process.29
Not all sintered diamond burs are the same. Depending on the manufacturer, the diamond particles can be natural or synthetic and can vary in chip size and shape as well as individual particle faceting.23 For example, the Diablo (Bredent, www.bredent.com) is a friction-grip sintered diamond bur that has an auto-sharpening property, according to the manufacturer. This means that as the diamond grit wears, it is automatically removed and replaced with a new diamond.
When comparing numerous diamond burs indicated for use on zirconia, Christensen30 notes the larger grit size instruments demonstrated more chatter and less control than medium and fine diamonds. According to information the author obtained from various manufacturers, their particular diamond fabrication was superior to their competition, the difference being in the quality of the diamond particle, the hardness of the binding material, and the precision and concentricity of the shaft. The longer the particle is embedded in the binding material, the more efficient the instrument’s cutting ability is. That could explain why some finer grit diamonds actually cut more efficiently than larger grit diamonds that have softer binding materials.
A literature search did not produce any scientific data that showed any one particular diamond bur, made specifically for zirconia, to be superior over another. The one point that all manufacturers share—no matter what diamond instrument is used—is that copious amounts of water, with very light or no pressure, should be used on zirconia. In other words, let the diamond bur do the work.
It is also recommended that the undersurface of the zirconia crown never be touched. This is because, as previously mentioned, all ground surfaces should be polished. It would be difficult to use polishing wheels inside the crown. Instead, the prepared tooth should be altered to completely seat the crown. More studies are needed to follow the handling of zirconia and the effects it can have on the long-term use of this material.
Gregg A. Helvey, DDS, is an adjunct associate professor at the Virginia Commonwealth University School of Dentistry in Richmond, Virginia and maintains a private practice in Middleburg, Virginia.
References
1. Vagkopoulou T, Koutayas SO, Koidis P, et al. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur J Esthet Dent. 2009;4(2):130-51.
2. Helvey GA. Zirconia and computer-aided design/computer-aided manufacturing (CAD/CAM) dentistry. Functional Esthet Rest Dent. 2007;Series J(3):28-39.
3. Christel P, Meunier A, Heller M, et al. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23(1):45-61.
4. Raigrodski AJ. Contemporary all-ceramic fixed partial dentures: a review. Dent Clin North Am. 2004;48(2):viii, 531-544.
5. Hauptmann H, Suttor D, Frank S, et al. Material properties of all-ceramic zirconia prosthesis [abstract 2910]. J Dent Res. 2000;79:507.
6. Rountree P, Nothdurft F, Pospiech P. In-vitro investigations on the fracture strength of all-ceramic posterior bridges of ZrO2-ceramic [abstract 173]. J Dent Res. 2001;80:57.
7. McLaren EA, Giordano RA. Zirconia-based ceramics: Material properties, esthetics, and layering techniques of a new veneering porcelain, VM9. Quintessence Dent Technol. 2005;28:99-111.
8. Helvey GA. Press-to-zirconia: a case study utilizing cad/cam technology and the wax injection method. Pract Proced Aesthet Dent. 2006;18(9):547-553.
9. Luthardt RG, Holzhüter MS, Rudolph H, et al. CAD/CAM-machining effects on Y-TZP zirconia. Dent Mater. 2004;20(7):655-662.
10. Garvie RC, Hannink RH, Pascoe RT. Ceramic steel? Nature.1975;258:703-704.
11. Kosmac T, Oblak C, Jevnikar P, et al. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent Mater. 1999;15(6):426-433.
12. Kosmac T, Oblak C, Jevnikar P, et al. Strength and reliability of surface treated Y-TZP dental ceramics. J Biomed Mater Res. 2000;53(4):304-313.
13. Gupta TK. Strengthening by surface damage in metastable tetragonal zirconia.J Am Ceram Soc.1980;63(1-2):117-21.
14. Green DJ. A technique for introducing surface compression into zirconia ceramics. J Am Ceram Soc. 1983;66(10):C178-C179.
15. Swain MV. Limitation of maximum strength of zirconia-toughened ceramics by transformation toughening increment. J Am Ceram Soc. 1985;68(4):C97-C99.
16. Ardlin BI. Transformation-toughened zirconia for dental inlays, crowns and bridges: chemical stability and effect of low-temperature aging on flexure strength and surface structure. Dent Mater. 2002;18(8):590-595.
17. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20(1):1-25.
18. Sato T, Shimada M. Transformation of yttria-doped tetragonal ZrO2 poly crystals by annealing under controlled humidity conditions. J Amer Ceram Soc. 1985;68(12):C320-C322.
19. Swab JJ. Low temperature degradation of Y-TZP materials. J Mater Sci. 1991;26(24):6706-6714.
20. Guazzato M, Quach L, Albakry M, et al. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J Dent. 2005;33(1):9-18.
21. Wang H, Aboushelib MN, Feilzer AJ. Strength influencing variables on CAD/CAM zirconia frameworks. Dent Mater. 2008;24(5):633-638.
22. Karakoca S, Yilmaz H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics. J Biomed Mater Res B Appl Biomater. 2009;91(2):930-937.
23. Giordano R. Boston University professor answers your zirconia questions. JDT Unbound. October 2008.
24. Xu HH, Kelly JR, Jahanmir S, et al. Enamel subsurface damage due to tooth preparation with diamonds. J Dent Res. 1997;76(10):1698-1706.
25. Yin L, Jahanmir S, Ives LK. Abrasive machining of porcelain and zirconia with a dental handpiece. Wear. 2003;255(7-12):975-989.
26. Yin L, Ives LK, Jahanmir S. Effects of fluids on the simulated clinical-dental machining of a glass ceramic. J Am Ceram Soc. 2004;87(1):173-175.
27. Yin L, Ives LK, Jahanmir S, et al. Abrasive machining of glass-infiltrated alumina with diamond burs. Machining Sci Technol. 2001;5(1):43-61.
28. Yin L, Song XF, Song YL, et al. An overview of in vitro abrasive finishing and CAD/CAM of bioceramics in restorative dentistry. Int J Mach Tool Manu. 2006;46(9):1013-1026.
29. Siegel SC, von Fraunhofer A. Dental cutting: the historical development of diamond burs. J Am Dent Assoc. 1998;129(6):740-745.
30. Christensen GJ. Zirconia cutting diamonds for crown removal. Clinician’s Report. August 2008.
About the Author
Gregg A. Helvey, DDS
Adjunct Associate Professor
Virginia Commonwealth University School of Dentistry
Richmond, Virginia
Private Practice
Middleburg, Virginia