Endodontic Obturation Considerations for the General Dentist
Larry Lopez
Endodontic obturation has been of interest to the dental profession for almost 200 years. During that time, many advances in materials and techniques have changed the approach to obturation. Regardless of the techniques or materials used, the goals of obturation have remained the same: to seal every portal of exit in the root canal system. It is not only apical openings that need to be sealed, but also lateral openings, coronal access openings, and, if they exist, perforations. This article reviews some of the techniques and materials used over the last two centuries and shows how they have contributed to the evolution of choices for obturation that are available to the contemporary clinician.
Hatton said, “Perhaps there is no technical operation in dentistry or surgery where so much depends on the conscientious adherence to high ideals as that of pulp canal filling.”1 Before 1800, when root canal fillings were done, the material was limited to gold. After that, obturations with various paraffins, oxychloride of zinc, and other metals and amalgams resulted in varying degrees of success.2 In 1847, Hill developed the first gutta-percha root canal filling material.3 Then, in 1867, Bowman demonstrated the first use of gutta-percha for canal filling in an extracted molar before the St. Louis Dental Society.3 In 1883, Perry demonstrated obturation using a gold wire wrapped in soft gutta-percha.4 S.S. White Technologies® Inc (Piscataway, NJ) began the manufacture and sale of gutta-percha points in 1887.5 Callahan demonstrated that gutta-percha could be softened and dissolved and used as a cementing agent through the use of rosins in 1914.6 Focusing on the concepts of focal infection, the hollow tube theory, and the concept that the primary cause of failure of root canal treatment was apical percolation of fluids and microorganisms, dental inventors have proposed many variations of the delivery of gutta-percha to the prepared canal.
The author believes that the most important steps in root canal therapy have to do with the cleaning, shaping, and disinfecting of the canal system. Studies have shown that in necrotic teeth it is virtually impossible to completely sterilize internal aspects of the root canal system.7 Advances in instrument technology have allowed the modern dentist to widen internal aspects of the root canal enough so that disinfectant solutions might reach the microorganisms that remain in the canal after physical debridement. Additionally, it has been shown that the use of ethylenediaminetetraacetic acid (EDTA) and sodium hypochlorite remove the smear layer and allow a more efficacious disinfection by irrigants8 (Figure 1).
ONE VISIT OR TWO?
There are basically two general situations encountered by the dental clinician performing root canal therapy: a vital pulp situation and a necrotic pulp situation. Root canal treatment can be completed in one visit if the pulp is vital. Obturation of patients whose condition is caused by a vital irreversible pulpitis may occur in one visit, even in the presence of acute pain.9 Necrotic teeth usually exhibit material contamination and may require a different approach to treatment.9 In the case of necrotic pulp tissues, with or without symptomatic apical pathosis, these cases may be treated in one visit based on what is known today, even if the patient presents with acute symptoms caused by acute periradicular abscess. However, the obturation procedure generally is delayed until the patient is asymptomatic for more easily managed possible complications.
In clinical studies, Sjögren et al10 thoroughly instrumented 55 infected teeth with apical pathosis using only 0.5% sodium hypochlorite. Before obturation, cultures were obtained using anaerobic techniques. After cleaning and shaping, bacteria could be detected in 22 teeth. Complete healing occurred in 94% of the cases that yielded a negative culture and the success rate of those showing positive cultures was only 68%. Other investigators were unable to confirm Sjögren’s results in a study of 39 patients with periapical lesions that had both positive and negative canal cultures at the time of obturation; in this study 21 teeth were treated in one visit and 18 teeth were treated in two visits, with an inter-appointment dressing of calcium hydroxide.11 Healing of the periapical area was observed over a period of 4.5 years, with complete healing occurring in 81% of the cases in the one-visit group and in 71% of the cases in the two-visit group. Generally, obturation can be performed in a single visit on necrotic teeth after the cleaning and shaping procedures if the canal can be dried and the patient is not experiencing swelling. An exception to this is the presence or persistence of exudates from the canal. One cannot obdurate a canal that cannot be dried. There are two points that should be reemphasized at this juncture.
In chronic cases that cannot be obturated in one visit, complete cleaning, shaping, and disinfection of the canal should be accomplished; calcium hydroxide or another antimicrobial agent should be placed temporarily in the root canal, and it should be sealed with a fluid barrier.12
In vital cases where no bacteria is present, it is important for the clinician not to introduce microorganisms into the canal by using aseptic techniques.9
THE LENGTH OF OBTURATION
The apical limit of root canal treatment obturation remains a controversy in endodontics even today. In this author’s exper-ience, the apical point of termination is 1 mm from the radiographic apex. Numerous studies have shown that there is a great deal of variability of the size and location of the foramen relative to the apex in the distance between the apical constriction and the radiographic apex.13,14 Another source of controversy is the existence and importance of accessory lateral canals. One study using scanning electron micrographs found that the number of accessory canals ranged from 1 to 16.15 Although lateral canals can be associated with pathosis, a recent study suggests that accessory canals are common, but may have little effect on periradicular pathosis.16
Several studies have suggested that the extrusion of obturation materials decreases the prognosis for complete regeneration of the periapical structures.17-20 One study21 examined root canals in an American population and found that periapical disease was found in 4.1% of all teeth and 31.3% of root-filled teeth. The study noted that periapical pathology was found in 43% of the teeth that had overfills of obturation materials. In another study examining 1,000 cases, the investigators found that overfilling resulted in a failure rate of 37%. They also found that this was four times greater than cases filled short.22 Yet another study found that in the necrotic teeth, better success was achieved when the procedures terminated at or within 2 mm of the radiographic apex. When the obturation material was shorter than 2 mm from the apex—or passed the apex as in extrusion cases—success rates were 20% lower in vital cases, and that terminating the obturation materials between 2 mm and 3 mm was acceptable.23 Another study suggests that in vital cases, terminating the obturation fill about 0.5 mm from the apex will have a higher success rate when compared with cases terminating the obturator more than 2 mm from the apex.24
TYPES OF SEALERS
Sealers are necessary to seal the space between the dental wall and the operating core material. Sealers also are necessary to fill the voids and all of the irregularities of the root canal system. Ideal properties of a root canal sealer may be summarized as:
- being biocompatible, non-inflammatory, and non-mutagenic.25
- providing good adhesion between itself, the canal wall, and the obturation material.
- providing a fluid barrier.
- being radiopaque so it can be seen on a radiograph.
- exhibiting inconsequential shrinkage on setting.
- non-staining to the tooth structure.
- exhibiting bacteriostasis.
- being slow-setting.
- being insoluble in tissue fluids.
- being relatively easy to remove in case of retreatment.
Zinc-oxide eugenol sealers have been widely used. Sealers comprised of calcium hydroxide were developed for therapeutic use. However, the antimicrobial and osteogenic activity that was hoped for has not been demonstrated. Glass ionomer sealers have been used because of their dentin bonding properties.26
In the 1960s, resin sealers were introduced. They do not contain eugenol and provide adhesion to the dental wall. AH-26 is a slow-setting epoxy resin that was found to release formaldehyde while setting. One in vivo study showed that after 8 days it was still not completely set.27 AH-plus is a modified epoxy resin in which the formaldehyde is not released. The sealing ability of AH-26 and AH-plus appear to be comparable.28 Newer resin sealers have been introduced that are of the methacrylate families (InnoEndo™, Heraeus Kulzer, Armonk, NY; Epiphany®, Pentron Clinical Technologies, Wallingford, CT; Real Seal™, Sybron Dental Specialties, Orange, CA; and EndoREZ®, Ultradent Products, Inc, South Jordan, UT) that show great promise for efficacious sealing of the canal system with high bio-tolerance. Because of their ability to penetrate dentin with resin tags, these new resin sealers appear to strengthen the root against vertical fracture.29
OBTURATION CORE MATERIALS
A wide variety of core materials and techniques is available to the contemporary clinician. However, regardless of the obturation technique chosen, emphasis should be placed on the process of cleaning, shaping, and disinfecting the canal system.
Gutta-Percha
Gutta-percha is the most popular core material. It is the trans-isomer of polyisoprene and two existing crystalline forms, alpha and beta.30 The unheated beta phase is a solid mass that is compactable. When heated, the material changes to the alpha crystal and becomes pliable and tacky and can be made to flow when pressure is applied. A disadvantage to the alpha phase is the shrinkage that occurs when the material sets.31 Current gutta-percha cones consist of approximately 65% zinc oxide, 20% gutta-percha, 10% radio-opacifiers, and 5% plasticizers.32 Unlike rubber, room temperature gutta-percha cannot be compressed and made to flow. Compaction results in the transmission of forces to the material and the canal wall, and may result in root fracture. Gutta-percha can be made to flow if it is modified by a heater or a solvent such as chloroform (Figure 2). This permits adaptation to the irregularities of the canal system. The alpha form of gutta-percha melts when heated above 65°C and when cooled extremely slowly, the alpha form recrystallizes.
Routine cooling results in the recrystallization to the beta form. When the alpha-phase gutta-percha is heated and cooled it undergoes less shrinkage, making it more dimensionally stable. Although gutta-percha points cannot be heat-sterilized, a recent study found that gutta-percha points should be sterilized before use by placing the cones in 5% sodium hypochloride for 1 minute.33 The study also found that 2% glutaraldehyde, 2% percent chlorhexidine, and 70% ethanol were not effective in killing the Bacillus subtilis spores.
Resilon
Recently, a new gutta-percha replacement material was introduced. This material, which is polyurethane that has been adapted for dental use, Resilon™ (Pentron Clinical Technologies) resembles gutta-percha, and can be placed as single cones, warm vertical compaction thermoplastic injection, or lateral compaction. The resin material is composed of polyester difunctional methacrylate resin, bioactive glass, and radiopaque fillers.
The advantage of the Resilon material is that it provides a chemical bond between the resin sealer used in its system and the core material (Figure 3). Studies have shown that it provides a superior seal coronally and apically with the bonding materials.34 The sealer used with Resilon is a resin matrix of bis-GMA, ethoxylated urethane dimethacrylate (UDMA), and hydrophylic difunctional methacrylates and fillers of calcium hydroxide. It also contains barium sulfate, barium glass, bismuth oxychloride, and silica. This sealer is used in combination with a primer/adhesive.
During the cleaning and shaping procedures, alternating rinses of EDTA and sodium hypochlorite remove the smear layer and open the dental tubules for penetration of the resinous sealer. Because resins will not set in the presence of oxygen, the oxygenating agent sodium hypochlorite should not be the last irrigant before the resin sealer is introduced into the canal. The last irrigant should be EDTA, followed by sterile water or 2% chlorhexidine solution. The Resilon core bonds to the resin sealer, which attaches to the etched root surface and forms a monobloc. This results in a better coronal seal and may strengthen the root against fracture. Investigators evaluated the coronal leakage of Resilon using Enterococcus faecalis and Streptococcus mutans in root canals filled using lateral and vertical compaction techniques with gutta-percha and AH-26 or Resilon and Epiphany sealer34 (Figure 4). The studies indicated that Resilon demonstrated significantly less coronal leakage when compared with gutta-percha. Another study, using a dog model to assess the ability of Resilon or gutta-percha and AH-26 in preventing apical periodontitis with microorganisms, showed periapical inflammation in 82% of roots obturated with gutta-percha and AH-26, while only 19% of roots obturated with the Resilon material showed such a response.35
Structural Obturators
In 2001, Pentron Clinical Technologies introduced the first obturator (FibreFill®) that would augment the restorative procedure. The company placed a single, precisely-sized, gutta-percha cone on the end of its highly successful resin fiber post, allowing the simultaneous placement of the obturator and a post (Figure 5). In 2005 Heraeus Kulzer introduced a similar system (InnoEndo) that used Resilon instead of gutta-percha as the obturation material. Both systems use a dual-cure resin sealer and an adhesive primer to seal the canal, similar to the methodologies used to seal cavity preparations in the coronal part of the tooth.
These systems appear to offer several advantages to the restorative dentist who is also performing the root canal. Investigators have found that they provide a bonded resin apical and coronal seal, which is superior to gutta-percha and AH-26 placed either laterally or as a warm vertical compaction36-38 (Figure 6). The obturator and sealer strengthen the root against fracture,39 and they augment the restorative procedure by helping to retain the core (Figure 7 and Figure 8). Because the obturators are placed passively, it eliminates any possibility of physical or thermal root strain, which could both lead to root fracture. The sealers are biocompatible, noninflammatory, and non-mutagenic.40 The InnoEndo system contains drill-less posts (same size and taper as 0.04 files), which eliminate the need to remove additional structure from the root, shortening the length of the procedure and safeguarding the root against fracture. The bond of the post to the radicular dentin is maximized, because there is no opportunity for obturation material to be burnished into the walls of the dentin that would minimize bonding sites.41
CONCLUSION
There are many good obturation systems available to the general dentist today. Studies suggest that the resin technologies hold promise for providing a superior seal without the possibility of stressing the root either physically or thermally.
REFERENCES
1. Hatton EH. Changes produced in the pulp and periapical regions, and their relationship to pulp canal treatment and to systemic disease. Dent Cosmos. 1924;66:1183.
2. Koch CRE: History of Dental Surgery. Vols 2 and 3. Ft. Wayne, Ind; National Art Publishing Co; 1909.
3. History Committee Missouri State Dental Association. History of Dentistry in Missouri. Fulton, Mo; Ovid Bell Press; 1938.
4. Perry SG. Preparing and filling the roots of teeth. Dent Cosmos. 1883;25(185).
5. Keane HC. A Century of Service to Dentistry. Philadelphia, Pa; SS White Dental Manufacturing Co; 1944.
6. Callahan JR. Rosin solution of the sealing of the dentinal tubuli and as an adjuvant in the filling of root canals. Journal of the Allied Dental Societies. 1914;9:53-63.
7. Walton RE. Histologic evaluation of different methods of enlarging the pulp canal. Int Endod J. 1976;2(10):304-311.
8. Hulsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int J Endodon. 2003;36(12):810-830.
9. Trope M, Bergenholtz G. Microbial basis for endodontic treatment: can a maximal outcome be achieved in one visit? Endodontic Topics. 2002;1(1): 40-53.
10. Sjogren U, Figdor D, Persson S, et al. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endodon J. 1997;30(5): 297-306.
11. Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endodon J. 2002;35:660-667.
12. Sjogren U, Hagglund B, Sundqvist G, et al. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16(10): 498-504.
13. Burch JG, Hulen S. The relationship of the apical foramen to the anatomic apex of the tooth root. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1972;34(2):262-268.
14. Chapman C. A microscopic study of the apical region of the human anterior tooth. J Br Endod Soc. 1969;3(4):52-58.
15. Gutierrez JH, Aguayo P. Apical foraminal openings in human teeth: number and location. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):769-777.
16. Barthel CR, Zimmer S, Trope M. Relationship of radiologic and histologic signs of inflammation in human root-filled teeth. J Endod. 2004;30(2):75-79.
17. Bergenholtz G, Lekholm U, Milthon R, et al. Retreatment of endodontic fillings. Scand J Dent Res. 1979;87(3):217-224.
18. Friedman S, Lost C, Zarrabian M, et al. Evaluation of success and failure after endodontic therapy using glass ionomer cement sealer. J Endod. 1995;21(7):384-390.
19. Seltzer S. Long-term radiographic and histological observations of endodontically treated teeth. J Endod. 1999;25(12):818-822.
20. Seltzer S, et al. Biologic aspects of endodontics. V. Periapical tissue reactions to root canal instrumentation beyond the apex and root canal fillings short of and beyond the apex. Oral Surg Oral Med Oral Pathol Radiol Endod. 1973;36(5):725-737.
21. Buckley M, Spangberg LS. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(1):92-100.
22. Swartz DB, Skidmore AE, Griffin JA Jr. Twenty years of endodontic success and failure. J Endod. 1983;9(5):198-202.
23. Wu MK, Wesselink PR, Walton RE. Apical terminus location of root canal treatment procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):99-103.
24. Peak JD, Hayes SJ, Bryant ST, et al. The outcome of root canal treatment: a retrospective study within the armed forces (Royal Air Force). Br Dent J. 2001;190(3):140-144.
25. Spangberg L. Biological effects of root canal filling materials. 6. The inhibitory effect of solubilised root canal filling materials on respiration of HeLa cells. Odontol Tidskr. 1969;77(2):121-131.
26. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endodon J. 1995;28(1): 12-18.
27. Allan NA. Setting times for endodontic sealers under clinical usage and in vitro conditions. J Endod. 2001;27:421-423.
28. De Moor RJ, De Bruyne MA. The long-term sealing ability of AH-26 and AH plus used with three gutta-percha obturation techniques. Quintessence Int. 2004;35(4): 326-321.
29. Teixeira FB, Teixeira E, Thompson JY, et al. Fracture resistance of roots endodontically treated with a new resin filling material. J Am Dent Assoc. 2004;135(5):646-652.
30. Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha. II. The history and molecular chemistry of gutta-percha. Oral Surg Oral Med Oral Pathol Radiol Endod. 1974;37(6): 954-961.
31. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha. Part V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg Oral Med Oral Pathol Radiol Endod. 1985;59(3):285-296.
32. Friedman CE, Sandrik JL, Heuer MA, et al. Composition and physical properties of gutta-percha endodontic filling materials. J Endod. 1977;3(8):304-308.
33. Siqueira JF Jr, da Silva CH, Cerqueira M das D, et al. Effectiveness of four chemical solutions in eliminating Bacillus Subtilis spores on gutta-percha cones. Endodon Dent Traumatol. 1998;14(3):124-126.
34. Shipper G, Orstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J Endodod. 2004;30(5): 342-347.
35. Shipper G, Teixeira FB, Arnold BS, et al. Periapical inflammation after coronal bacterial inoculation of dog roots filled with gutta-percha or Resilon. J Endod. 2005;31:91.
36. Shipper G, Trope M. In vitro microbial leakage of endodontically treated teeth using new and standard obturation techniques. J Endod. 2004;30(3):154-158.
37. Platt J, Duke S, Moore K, et al. An in vitro evaluation of a novel endodontic obturation system. University of Indiana Dental School. Unpublished study.
38. Kim S, Chang P, Iqbal M. University of Pennsylvania School of Dental Medicine. A comparison of coronal and apical dye leakage with two obturation systems. From abstract submitted to AAE meeting; 2002.
39. Ferrari M. University of Sienna. Fracture strength of endodntically treated monoradicular teeth with a new endodontic obturating system (Fibrefill): an vitro study. Un-published study.
40. Thongthammachat S, Platt JA, Katona TR, et al. Fracture resistance and fracture resistance after fatigue loading of 4 different post and cores in endodontically treated teeth. Compend Contin Educ Dent. 2006;27(7):340-346.
41. Serafino C, Gallina G, Cumbo E, et al. Surface debris of canal walls after post space preparation in endodontically treated teeth: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):381-387.
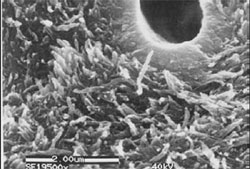 | 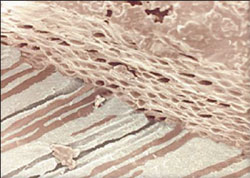 |
| Figure 1 SEM showing removal of smear layer following irrigation with NaOCL and EDTA. | Figure 2 Thermoplastized gutta-percha shown within the dentinal tubules. |
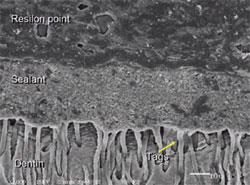 | 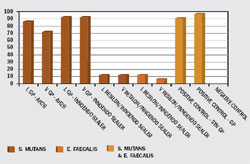 |
| Figure 3 Micrograph demonstrating intimate contact of sealer and Resilon, a synthetic guttapercha alternative. | Figure 4 Coronal leakage study comparing various obturation materials. |
 | 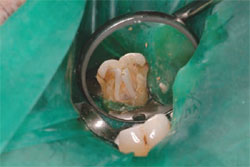 |
| Figure 5 InnoEndo resin sealer pictured with automix tips and syringe. | Figure 6 Build-up performed at endodontic appointment. |
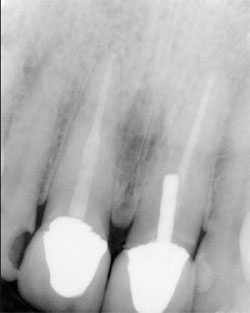 | 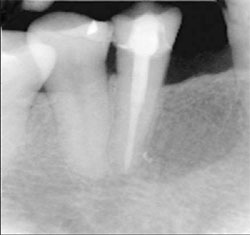 |
| Figure 7 Maxillary right central incisor treated with an endodontic fiber obturator, decreasing the possibilty of perforation with a seperate post preparation. | Figure 8 Mandibular right premolar treated with an endodontic fiber obturator and restoration completed. |
| About the Author | |
Larry Lopez, DDS | |




