Adhesive Considerations in the Placement of Direct Composite Restorations
Gary Alex, DMD
Abstract
The late Dr. John Gwinnett, one of the most brilliant and respected members of the dental research and teaching community, often characterized bonding as a chain, a series of links between the restorative material being placed and the tooth tissues.1 And as in a chain, the bond of a restorative material to the tooth substrate is only as strong as its weakest link. Dr. Gwinnett inspired a new generation of clinicians and researchers, the author included, to think about adhesion in an entirely new fashion. Insights and innovations by contemporaries, friends, and colleagues of Dr. Gwinnett, including Byoung Suh, John Kanca, Charlie Cox, Franklyn Tay, David Pashley, Sumita Mitra, Wayne Barkmeier, and many others, also advanced our knowledge and understanding of adhesion. This eventually led to the development of many of the various adhesive techniques and materials utilized in dentistry today. This paper will focus on some of the current concepts and techniques developed to manage the adhesive interface during the placement of direct posterior composite restorations.
Direct composite resins have the potential to offer a reasonably predictable alternative to amalgam and other metal-based restoratives. This assumes they are utilized in the appropriate clinical situation and are properly placed. In fact, the increasing demand for tooth-colored restorations, conservation of tooth structure, and cosmetic dental procedures has encouraged the widespread placement of direct composite restorations.2-4 The greater level of clinical success with direct composites is most likely related to material developments, improved clinical skills and techniques, and dramatic advances in adhesive technology.5 Since the use of directly placed composites is a mainstay in the majority of restorative practices, it is imperative that dentists understand the rationale for specific clinical techniques, as well as material idiosyncrasies, in order to optimize the adhesive interface between the composite restorative and the tooth substrate.
The majority of direct composites utilized in restorative dentistry today consists of a methacrylated resin matrix (ie, usually a blend of several resins) that is mixed with various glass filler particles, pigments, stabilizers, and chemical and/or light activated initiators. The filler particles in composites are typically silanated. Silane serves as a coupling agent between organics (ie, the resin matrix) and inorganics (ie, the glass fillers). Filler particles can be manufactured in various shapes and sizes and from any number of inorganic glasses (eg, silica, zirconia-silica, barium silicate, lithium, strontium, and ytterbium). As a generalization, larger particle, more heavily filled composites have superior physical properties while smaller particle composites, such as microfills, are not as strong but have a tendency to wear and polish better.6 While an in-depth discussion and characterization of composite chemistry and classification is beyond the scope of this particular paper, clinicians should be aware that the specific nature of the composite being utilized has a direct bearing on what is occurring at the adhesive interface. For example, all current composite formulations shrink to some degree during polymerization (ie, generally 1.5% to 5% by volume). The total amount of shrinkage, the rate of shrinkage, and the elastic modulus (ie, stiffness) of the composite are just some of the factors that influence the degree of stress and strain (ie, deformation) induced at the adhesive interface during composite polymerization.
When placing a direct composite, the actual linkage between composite and the tooth tissues is usually mediated through the use of a dentin bonding agent. The development of practical adhesive dentistry can be traced to Michael Buonocore who, in 1955, discovered he could increase the retention of acrylic-based restoratives by first treating the teeth with phosphoric acid.7 Subsequent research by Buonocore, Gwinnett, and Matsui elucidated the mechanism of adhesion between enamel and resin restoratives via resin tag formation.8 Long-term bonding to phosphoric acid-etched enamel surfaces has proven to be highly reliable and predictable; long-term bonding to dentin is not as predictable, regardless of the dentin bonding agent used. Clinicians can generally bond predictably to enamel, but not nearly as predictably to dentin because of the morphologic, histologic, and compositional differences between the two substrates.9 For one thing, dentin is a highly variable substrate. Superficial, middle, and deep dentin can vary significantly in their structural and chemical composition. Enamel, on the other hand, is quite consistent throughout and is also considerably more mineralized than dentin. The inorganic content of mature enamel is approximately 96% hydroxyapatite by weight; the remainder consists of water and organic material. Dentin, on the other hand, is approximately 70% hydroxyapatite by weight, 18% organic material (ie, predominantly collagen), and 12% water.1,10 These percentages are not consistent and can vary significantly depending on a number of factors, including dentin depth, age of the teeth, and history of tooth trauma and/or pathology. This, coupled with the relatively high water content of dentin, presents a significant challenge for consistent and reliable long-term bonding. However, this does not mean that stable and strong initial bonds to dentin cannot be attained. Laboratory studies have shown that many current adhesive systems are capable of producing bond strengths to dentin that equal or surpass those of acid-etched enamel controls.
The problem is that most of these are short-term studies (ie, often 24 hours) and the focus needs to be on long-term studies. It is of concern that the literature is replete with longer-term studies—both in vitro and in vivo—that demonstrate a worrisome trend toward eventual degradation of the dentin/adhesive interface.11-18 This could contribute to the observed clinical problem of porcelain veneers sometimes debonding over time when preparations are largely in dentin. Rarely is veneer debonding a problem when significant amounts of enamel remain.19 Microleakage, nanoleakage, hydrolysis, dentin permeability, pulpal pressure, shrinkage stress, "water tree" formation, insufficient hybrid layer formation, phase separation, dentin tubule orientation, occlusion, enzymes released by bacteria, and operator error have all been implicated as potential causes of deterioration of the dentin/adhesive interface over time.20-25 When placing a direct composite, it makes sense to utilize techniques and materials that, hopefully, will increase their long-term predictability.
Know Your Adhesive System
All dentin bonding systems employ acids of one type or another to facilitate adhesion to the tooth tissues. Acidic treatment of dentin and/or enamel creates a zone of demineralization, which is subsequently (ie, total-etch) or concurrently (ie, self-etch) infiltrated with various bifunctional primers and resins. While many adhesive systems are capable of providing acceptable clinical results if used in a knowledgeable fashion with attention to detail, all have their particular idiosyncrasies.
The fourth generation, or three-step total-etch systems,a-c generally have good long-term clinical track records and are perhaps the most versatile of all the adhesive categories because they can be employed for virtually any bonding protocol (ie, direct, indirect, self-cure, dual-cure, light-cure). These systems are still the "gold standard" by which the newer systems are judged. Indeed, none of the newer systems in the marketplace today perform any better, and often perform worse, than the original multiple component total-etch systems of 15 years ago if bond strength to dentin/enamel, microleakage, and long-term durability are used as the evaluation criteria.16
The fifth generation, or two-step total-etch systems,d-i evolved from the desire to simplify the three-step total-etch system protocol. As a group, these are among the most popular systems presently being utilized in dentistry. They have generally proven to be highly effective, simpler, and faster than their multiple component predecessors. On the down side, many in this category, albeit with some exceptions, are not as predictable as the three-step total-etch systems when it comes to bonding to self- and dual-cure composites.26 In addition, the two-step total-etch systems may be more susceptible to water degradation over time than three-step total-etch systems.27 This is because the polymerized primer of the two-step systems tends to be hydrophilic in nature. When using a three-step system, the hydrophilic primer is covered by a more hydrophobic resin, making it less susceptible to water sorption.21
If the clinician elects to utilize a fourth and fifth generation total-etch system in the placement of a direct composite, he or she needs to be aware that the majority of laboratory studies show that these types of systems perform best when placed on moist dentin. This has been termed "wet" bonding,28-30 although moist bonding may be a more accurate description of the phenomena. Dentin exposed to phosphoric acid results in dissolution of the inorganic hydroxyapatite matrix. As the matrix dissolves, the collagen fibrils, which are inherent in dentin, become exposed as they are no longer supported and surrounded by their inorganic scaffolding. It is this friable "collagen network" that must be infiltrated by subsequently placed primers and resins to ensure good bonding. Air-drying of acid-etched dentin causes collapse of the collagen network and interferes with subsequent primer/resin infiltration.31 In dentin that is left moist (eg, after acid conditioning), the collagen fibrils remain in a relatively "open" state and appear to be more permeable to subsequently placed primers and resins. The author's recommended technique when utilizing a total-etch protocol on unlined dentin is not to air-dry the dentin once the phosphoric acid conditioner is washed off. The excess water is simply blotted out with cotton pellets prior to placing the primer. This results in a visibly moist dentin surface instead of "puddles" of water, which should be avoided.32,33 It warrants noting that some total-etch systems, usually those that are acetone-based, appear to be more sensitive to this wet/dry dentin issue than others (eg, alcohol/water based systems). This highlights an inherent ambiguity many have with the concept of wet bonding, namely exactly how wet is wet?
Perhaps the biggest advantage of the sixth generation, or two-component, self-etching systemsj-n is that their efficacy appears to be less dependent on the hydration state of the dentin than total-etch systems. Since the dentin is not pretreated with phosphoric acid—as is the case with total-etch systems—no exposed collagen layer is present to collapse on air-drying prior to placement of the self-etching primers. Clinically this means that "wet" bonding is not a concern and the tooth surface can be briefly air-dried prior to placing a self-etching primer. This is not to say that self-etch systems perform any better than total-etch systems, but they seem to be less technique sensitive in this regard. One could also argue that a possible advantage of a self-etching system is that demineralization of the dentin occurs concurrently with primer infiltration. In principle this helps ensure that the entire zone of demineralization is saturated with primer where it can then be polymerized in situ. On the down side, many products in this category do not etch enamel as well as their total-etch cousins34 and many are not compatible with self- and dual-cure composites.26 A common clinical technique reported by many employing one of the popular self-etching systemsj is to first etch the enamel with traditional phosphoric acid prior to using it. This helps ensure good bond strength to enamel but it does require an additional step in the bonding protocol. Those utilizing this technique should take care to confine the phosphoric acid solely to the enamel. Additional etching of the dentin with phosphoric acid could, in principle, create an "over-etch" situation where the demineralization zone is too deep for subsequently placed primers to completely penetrate.
The seventh generation, or one-bottle self-etching systems,o-s represents the latest simplification of adhesive systems. With these systems all the ingredients required for bonding are placed in, and delivered from, a single bottle. This greatly simplifies the bonding protocol. However, the price for simplification may be compromise. Incorporating and placing all of the chemistry required for a viable adhesive system into a single bottle, and having it remain stable over a reasonable period of time, poses a significant challenge. These inherently acidic systems tend to have a significant amount of water in their formulations and may be prone to hydrolysis and chemical breakdown.35,36 In addition, once placed and polymerized, they are generally more hydrophilic than two-step self-etching systems, which makes them more prone to water sorption.37 This could contribute to hydrolysis and degradation of the adhesive interface,38 as well as a reduction in mechanical properties of the composite restorative.39 The acidic nature of the polymerized primers in seventh generation adhesives generally makes them unsuitable for use with self-cure composites since their acidic nature degrades the tertiary aromatic amines required for chemical polymerization of self-cure composites.37,40,41 It is this author's opinion that while offering ease and simplicity, seventh generation adhesive systems should be used cautiously until more independent research clearly demonstrates their short- and long-term effectiveness.
In principle, the "ideal" adhesive system would be one that is hydrophilic when first placed in order to interact with dentin, which inherently has a high water content, but then becomes completely hydrophobic once polymerized in order to discourage water sorption and hydrolysis. Unfortunately, no such chemistry currently exists. A new, recently introduced total-etch systemt is among the first to address this issue by utilizing chemistries that are less hydrophilic in nature.
Flowable Composite Liners
One common clinical technique thought to improve the performance of direct composite restorations is the use of flowable composites. With this technique a flowable (ie, a lightly filled composite) is placed in a thin layer after a dentin bonding agent is placed but prior to placement of a more heavily filled composite restorative. In principle, flowable composites, by virtue of their low viscosity, are able to get into the "nooks and crannies" of the preparation. This helps ensure optimal adaptation to the previously placed dentin bonding agent, as well as to the higher viscosity composite restorative that is subsequently placed. One could also argue that flowable composites, having a relatively low modulus of elasticity, are able to act as stress-reducing liners during polymerization and shrinkage of the subsequently placed composite.42 The literature is equivocal regarding the use of flowables under direct composite restorations in terms of reducing microleakage, with some studies strongly supporting this technique and others showing no benefit.43-50 Regardless of the research, many dentists have used the technique with generally good success.
RMGI Liners
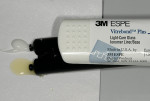
Resin modified glass ionomer (RMGI) liners represent an alternative and, in this author's opinion, possibly better option to flowable composite liners.51 With this technique a RMGI lineru is placed on the dentin in a thin layer prior to placing a dentin bonding agent and composite restorative. RMGI liners have several potential advantages over flowable composites. RMGI liners have the intrinsic ability to both micromechanically and chemically interact with dentin.52 They are simple to mix and place, release high sustained levels of fluoride,53 have significant antimicrobial properties,54,55 evidence very low solubility,56,57 and exhibit a favorable modulus of elasticity and coefficient of thermal expansion and contraction similar to that of dentin.58 One of the most important characteristics of liners in regard to direct composites may be their potential to act as stress-absorbing "buffers" during polymerization shrinkage of the composite restorative. Both flowable composite and RMGI liners have a low modulus of elasticity and the ability to deform and/or flex to a degree when subjected to an external force. This characteristic is thought to attenuate shrinkage stress from the subsequently placed higher modulus composite restorative although, in the case of flowable composites, at least one study showed this to be product specific.59 Another study showed that when MOD composite restorations were placed in cavities lined with either a flowable compositev or a RMGI linerw that the RMGI group had significantly less cusp deformation due to polymerization shrinkage.60 This could be because the modulus of a RMGI is less than a flowable, therefore more flexible, and/or the fact that RMGI liners undergo hygroscopic expansion once polymerized, which may compensate for some of the polymerization shrinkage.61,62 In addition, the success of a flowable composite's bond to dentin is highly contingent on the operator's ability to first correctly place a dentin bonding agent. A RMGI liner is placed prior to placing a dentin bonding agent, making it much less technique sensitive in this regard. Many clinicians have anecdotally reported a significant reduction in postoperative sensitivity after switching to RMGI liners.62,63 The author considers that the utilization of RMGI liners is also the simplest and most predictable method for managing microleakage/nanoleakage under direct composites. The literature is replete with both in vivo and in vitro studies supporting this belief.64-76 Many of these studies directly compare the use of flowable liners vs. RMGI liners in terms of controlling microleakage. The author has been unable to find any study where flowable composites, in conjunction with a dentin bonding agent, outperform RMGI liners in this regard.
Don't Forget the Small Details—WHICH REALLY AREN'T THAT SMALL
- Check the expiration date! Dentin bonding agents utilize chemistries that can deteriorate significantly over time. Refrigeration may improve shelf-life, but the dentin bonding agent should be removed from the refrigerator 30 minutes prior to use because some may not perform as well when cold.77
- Make sure to evaporate the solvents. All adhesive systems employ acetone, ethanol, water, or a combination of these as solvents for their particular monomers. It is very important to evaporate these solvents by air-drying for an adequate period of time prior to polymerization. Inadequate solvent evaporation results in incomplete resin polymerization and can contribute to leakage and breakdown of the adhesive interface.78
- Periodically check the bonding light. Light tips may become scratched or damaged, bulbs can lose intensity, and filters can crack. All of these can significantly reduce energy output. It is sound policy to regularly test the energy output of bonding lights with a radiometer.
- If all else fails, read the directions. Every adhesive system, even those in the same generation, has specific placement idiosyncrasies that must be precisely followed for optimal results. What works well for one system may not be applicable for another system.
The author's personal preference in the placement of direct composites is to utilize a two-step total-etch systemd in conjunction with a RMGI lineru. The entire issue of wet bonding becomes less of a concern with this technique because a significant portion of the exposed dentin is covered by the RMGI liner prior to placing the phosphoric acid and resin primer.
The clinical technique is described below:
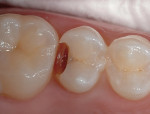
1. After the preparation is complete, clean the dentin and enamel surfaces with slurry of fine pumice and water using pumicex on a small brushy (Figure 1 and Figure 2). The use of an antimicrobial solution is not recommended because in-house corporate studies have shown some of these solutions can have an adverse effect on the adhesion of the RMGI, which will be placed next.79 In any case, the RMGI to be placed is antimicrobial.
2. Wash thoroughly with an air/water spray; quickly air-dry.
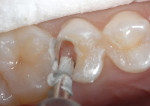
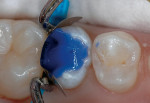
3. Mix and place a thin layer of the RMGI lineru. Note: the author generally covers most of the exposed dentin up to the dentoenamel junction (Figure 3 and Figure 4). Light polymerize for 20 seconds. When placing a RMGI line in this manner, the principle of "wet" bonding using a total-etch system is no longer a major factor because most of the dentin has already been covered by the RMGI.
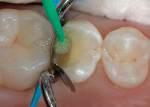
4. Place phosphoric acid on all enamel margins for 10 seconds; fill the entire preparation with phosphoric acid for another 10 seconds (Figure 5 and Figure 6). Wash thoroughly with air/water spray. Briefly air-dry. Blot drying is not required if most of the dentin has been covered by the RMGI.
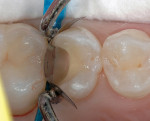
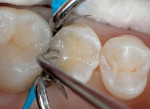
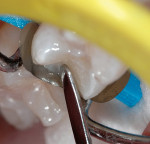
5. Place several liberal coats of a two-step total-etch systemd (Figure 7 and Figure 8) and air-dry for a minimum of 10 seconds. Light-cure for 10 seconds.
6. Place a nanofilled compositez utilizing a horizontal placement technique for the dentin increments and a lateral placement technique for the enamel increments (Figure 9 and Figure 10). Cure each increment for 10 seconds.
7. Finish and polish the completed restoration (Figure 11 and Figure 12).
Conclusion
Proper management of the adhesive interface is crucial for the predictable placement of direct composites. This requires an understanding of the materials being utilized, the substrate being bonded to, and a correct and precise clinical protocol. It is up to clinicians to objectively examine their own clinical techniques, as well as successes and failures, in order to determine if a change in materials and/or protocol is warranted.
References
1. Gwinnett AJ. Bonding basics: What every clinician should know. Esthetic Dent Update. 1994;5:35-41.
2. Cosmetic dental services expanding. Dent Prod Rep. 1998;1:34.
3. Allen EP, Brodine AH, Cronin RJ Jr, et al. Annual review of selected dental literature: report of the Committee on Scientific Investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 2005;94:146-176.
4. Baratieri LN, Ritter AV. Four-year clinical evaluation of posterior resin-based composite restorations placed using the total-etch technique. J Esthet Restor Dent. 2001;13:50-57.
5. Alex G. Adhesive dentistry: where are we today? Compend Contin Educ Dent. 2005;26:150-155.
6. Ferreira S, Kugel G, Martin S, et al. Direct esthetic adhesive restorative materials. Inside Dentistry. 2006;2:48-51.
7. Buonocore MG. A simple method of increasing the adhesion of acrylic filling to enamel surfaces. J Dent Res. 1955;34:849-853.
8. Buonocore MG, Gwinnett AJ, Matsui A. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch Oral Biol. 1968;13:61-70.
9. Alex G. Adhesive dentistry in the new millennium. Oral Health. 2000;59-64.
10. Van Meerbeek B, Lambrechts P, Inokoshi S, et al. Factors affecting adhesion to mineralized tissues. Oper Dent. 1992;Suppl 5:111-124.
11. Hashimoto M, Ohno H, Kaga M, et al. Resin-tooth adhesive interfaces after long-term function. Am J Dent. 2001;14:211-215.
12. Gwinnett AJ, Yu S. Effect of long-term water storage on dentin bonding. Am J Dent. 1995;8:109-111.
13. Hashimoto M, Ohno H, Kaga M, et al. In-vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79:1385-1391.
14. Meiers JC, Young D. Two-year composite/dentin bond stability. Am J Dent. 2001;14:141-144.
15. Okuda M, Pereira PN, Nakajima M, et al. Relationship between nanoleakage and long-term durability of dentin bonds. Oper Dent. 2001:26;482-490.
16. De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118-132.
17. Koshiro K, Inoue S, Tanaka T, et al. In-vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. Eur J Oral Sci. 2004: 112:368-375.
18. Carrilho MR, Carvalho RM, Tay FR, et al. Durability of resin-dentin bonds related to water and oil storage. Am J Dent. 2005;18:315-319.
19. Christensen GJ. Are veneers conservative treatment? J Am Dent Assoc. 2006; 137:1721-1723.
20. Tay FR, Frankenberger R, Krejci I, et al. Single-bottle adhesives behave as permeable membranes after polymerization. I. In vivo evidence. J Dent. 2004;32:611-621.
21. Hashimoto M, Ito S, Tay FR, et al. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;83:843-848.
22. Santerre JP, Shajii L, Leung BW. Relationship of dental composite formulations to their degradation and release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136-151.
23. Pashley DH, Tay FR, Yiu C, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216-221.
24. Finger WJ, Balkenhol M. Practitioner variability effects on dentin bonding with an acetone-based one-bottle adhesive. J Adhes Dent. 1999;1:311-314.
25. Purk JH, Dusevich V, Glaros A, et al. In vivo versus in vitro microtensile bond strength of axial versus gingival cavity preparation walls in Class II resin-based composite restorations. J Am Dent Assoc. 2004;135:185-193.
26. CRA Newsletter. 2003;27(4):2-3.
27. De Munck J, Van Meerbeek B, Yoshida Y, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136-140.
28. Kanca J. Improving bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:35-42.
29. Kanca J. Effect of resin primer solvents and surface wetness on resin composite bond strength to dentin. Am J Dent. 1992;5:213-215.
30. Gwinnett AJ. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992;5:127-131.
31. Gwinnett AJ. Chemically conditioned dentin: a comparison of conventional and environmental scanning electron microscopy findings. Dent Mater. 1994;10:150-155.
32. Tay FR, Gwinnett AJ, Wei SH. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free acetone-based, single-bottle primer/adhesives. Dent Mater. 1996;12:236-244.
33. Pereira GD, Paulillo LA, De Goes MF, et al. How wet should dentin be? Comparison of methods to remove excess water during moist bonding. J Adhes Dent. 2001;3:257-264.
34. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives. Part II: etching effects on unground enamel. Dent Mater. 2001;17:430-444.
35. Nishiyama N, Tay FR, Fujita K, et al. Hydrolysis of functional monomers in single-bottle self-etching primer-correlation of 13C NMR and TEM findings. J Dent Res. 2006;85: 422-426.
36. Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21:895-910.
37. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? Can Dent Assoc. 2003;69:726-731.
38. Pashley DH. The evolution of dentin bonding. Dent Today. 2003;22:112-114.
39. Bastioli C, Romano G, Migliaresi C. Water sorption and mechanical properties of dental composites. Biomaterials. 1990;11;219-223.
40. Sanares AM, King NM, Itthagarun A, et al. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent Mater. 2001;17:542-556.
41. Suh BI, Feng L, Pashley DH, et al. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J Adhes Dent. 2003;5:267-282.
42. Terry DA, Leinfelder KF. Managing stress with composite resin, Part 1. The restorative-tooth interface. Dent Today. 2006;25:98-104.
43. Frankenberger R, Kramer N, Pelka M, et al. Internal adaptation and overhang formation of direct Class II resin composite restorations. Clin Oral Investig. 1999;3:208-215.
44. Chuang SF, Jin YT, Liu JK, et al. Influence of flowable composite lining thickness on Class II composite restorations. Oper Dent. 2004;29:301-308.
45. Perdigao J, Anauate-Netto C, Carmo AR, et al. The effect of adhesive and flowable composite on postoperative sensitivity: 2-week results. Quintessence Int. 2004;35:777-784.
46. Attar N, Turgut MD, Gungor HC. The effect of flowable resin composites as gingival increments on the microleakage of posterior resin composites. Oper Dent. 2004;29:162-167.
47 Leevailoj C, Cochran MA, Matis BA, et al. Microleakage of posterior packable resin composites with and without flowable liners. Oper Dent. 2001;26:302-307.
48. Jain P, Belcher M. Microleakage of Class II resin-based composite restorations with flowable composite in the proximal box. Am J Dent. 2000;13:235-238.
49. Hagge M, Lindemuth JS, Mason JF, et al. Effect of four intermediate layer treatments on microleakage of Class II composite restorations. Gen Dent. 2001; 49:489-495.
50. Wibowo G, Stockton L. Microleakage of Class II composite restorations. Am J Dent. 2001;14:177-185.
51. Alex G. The use of resin-modified glass ionomer liners under composite resins: Should they be used to help control microleakage? Inside Dentistry. 2005;1:30-33.
52. Yoshida Y, Van Meerbeek B, Nakayama Y, et al. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79:709-714.
53. Mitra SB. In vitro fluoride release from a light-cured glass-ionomer liner/base. J Dent Res. 1991;70:75-78.
54. DeSchepper EJ, Thrasher MR, Thurmond BA. Antibacterial effects of light-cured liners. Am J Dent. 1989;2:74-76.
55. Scherer W, Lippman N, Kaim J, et al. Antimicrobial properties of VLC liners. J Esthet Dent. 1990;2:31-32.
56. Powell LV, Gordon GE, Johnson GH. Clinical comparison of Class V composite and glass ionomer restorations. Am J Dent. 1992;5:249-252.
57. Croll TP. Visible light-hardened glass-ionomer cement base/liner as an interim restorative material. Quintessence Int. 1991;22:137-141.
58. Mitra SB, Conway WT. Coefficient of thermal expansion of some methacrylate modified glass ionomers. J Dent Res. 1994;73:218. Abstract #944.
59. Braga RR, Hilton TJ, Ferracane JL. Contraction stress of flowable composite materials and their efficacy as stress-relieving layers. J Am Dent Assoc. 2003; 134:721-728.
60. Alomari QD, Reinhardt JW, Boyer DB. Effect of liners on cusp deflection and gap formation in composite restorations. Oper Dent. 2001;26:406-411.
61. Leinfelder KF, Freedman G, Pakroo JS. Postoperative sensitivity: bonded cavity liners revisited. Dent Today. 2001;20:82-87.
62. Ruiz JL, Mitra S. Using cavity liners with direct posterior composite restorations. Compend Contin Educ Dent. 2006;27:347-351.
63. Christensen G. Reducing postoperative sensitivity in Class I and Class II resin restorations. Dent Prod Report. 2001;February:94-96.
64. Akpata ES, Sadiq W. Post-operative sensitivity in glass-ionomer versus adhesive resin-lined posterior composites. Am J Dent. 2001;14:34-38.
65. Sidhu SK, Henderson LJ. In vitro marginal leakage of cervical composite restorations lined with a light-cured glass ionomer. Oper Dent. 1992;17:7-12.
66.Gupta S, Khinda VI, Grewal N. A comparative study of microleakage below cemento-enamel junction using light cure and chemically cured glass ionomer cement liners. J Indian Soc Pedod Prev Dent. 2002;20:158-164.
67. Aboushala A, Kugel G, Hurley E. Class II composite resin restorations using glass-ionomer liners: microleakage studies. J Clin Pediatr Dent. 1996;21:67-70.
68. Dietrich T, Kraemer M, Losche GM, et al. Influence of dentin conditioning and contamination of the marginal integrity of sandwich Class II restorations. Oper Dent. 2000;25:401-410.
69. Murray PE, About I, Franquin JC, et al. Restorative pulpal and repair responses. J Am Dent Assoc. 2001;132:482-491.
70. Beznos C. Microleakage at the cervical margin of composite Class II cavities with different restorative techniques. Oper Dent. 2001;26:60-69.
71. Besnault C, Attal JP. Simulated oral environment and microleakage of Class II resin-based composite and sandwich restorations. Am J Dent. 2003;16:186-190.
72. Aboush YE, Torabzadeh H. Clinical performance of Class II restorations in which resin composite is laminated over resin-modified glass ionomers. Oper Dent. 2000;25:367-373.
73. Retief DH, McCaghren RA, Russell CM. Microleakage of Vitrebond/P-50 Class II restorations. Am J Dent. 1992;5:130-132.
74. Sidhu SK, Henderson L. Microleakage of cervical composite restorations lined with light-cured glass ionomers. J Dent Res. 1990;69:945. Abstract #102.
75. Loguercio AD, Alessandra R, Mazzocco KC, et al. Microleakage in class II composite resin restorations: total bonding and open sandwich technique. J Adhes Dent. 2002:4;137-144.
76. Guan J, Williams PT, Stockton L. Microleakage in Class II composite restorations. J Dent Res. 2002:81 (Special Issue A): Abstract #3360.
77. Sundfeld RH, da Silva AM, Croll TP, et al. The effect of temperature on self-etching adhesive penetration. Compend Contin Educ Dent. 2006;27:552-557.
78. Hashimoto M, Tay FR, Svizero NR, et al. The effects of common errors on sealing ability of total-etch adhesives. Dent Mater. 2006;22:560-568.
79. Mitra SB. Personal conversation.
a All-Bond 2®, BISCO, Inc., Schaumburg, IL
b Scotchbond™ Multi-purpose Plus, 3M™ ESPE™, St. Paul, MN
c OptiBond® FL, Kerr Corporation, Orange, CA
d One-Step® Plus, BISCO, Inc., Schaumburg, IL
e Adper™ Single Bond Plus, 3M™ ESPE™, St. Paul, MN
f OptiBond® Solo, Kerr Corporation, Orange, CA
g Prime&Bond® NT, DENTSPLY Caulk, Milford, DE
h Excite®, Ivoclar Vivadent, Amherst, NY
i One Coat® Bond, Coltène/Whaledent, Cuyahoga Falls, OH
j Clearfil™ SE Bond, Kuraray America, Inc., New York, NY
k Simplicity™, Apex Dental Materials, Inc., Deer Park, IL
l All-Bond SE™ SPE, BISCO, Inc., Schaumburg, IL
m Adper™ Prompt™ L-Pop™, 3M™ ESPE™, St. Paul, MN
n One Coat® Self-Etching Bond, Coltène/Whaledent, Cuyahoga Falls, OH
o I-Bond™, Heraeus Kulzer, Armonk, NY
p G-Bond™, GC America, Inc., Alsip, IL
q Xeno® IV, DENSPLY Caulk Milford, DE
r Clearful® S3 Bond, Kuraray America, Inc., New York, NY
s OptiBond® All-In-One, Kerr Corporation, Orange, CA
t All-Bond 3™, BISCO, Inc., Schaumburg, IL
u Vitrebond™ Plus, 3M™ ESPE™, St. Paul, MN
v Revolution®, Kerr Corporation, Orange, CA
w Vitrebond™, 3M™ ESPE™, St. Paul, MN
x Preppies™, Ultradent Products, Inc., South Jordan, UT
y ICB® brush, Ultradent Products, Inc., South Jordan, UT
z Filtek™ Supreme Plus, 3M™ ESPE™, St. Paul, MN
About the Author
Gary Alex, DMD
Co-Director, Long Island Center for Dental Esthetics and Occlusion
Long Island, New York
Private Practice
Huntington, New York