Effectiveness of a Combined In-Office and Take-Home Whitening System for Teeth Shades A3.5 to A4
Abstract:
As teeth whitening increases in popularity, dental professionals are challenged to select the proper materials and technique to create esthetically whitened smiles for their patients. This study examined nine subjects with baseline shades of A3.5 to A4 on four to six of their maxillary anterior teeth with the VITA Classical (VC) shade guide. All subjects received one session of in-office whitening (Philips Zoom WhiteSpeed), 3 weeks of at-home tray whitening (Zoom NiteWhite), and the option of a second WhiteSpeed treatment. Subjects achieved, on average, documented change of 11.1 shades. Only two subjects reported any sensitivity, thereby demonstrating the effectiveness of a combined whitening system, including both chairside and take-home whitening, and a sensitivity prevention protocol.
With an increasing emphasis on esthetics in recent years, tooth whitening has become a popular dental procedure and dental practice service.1 Tooth-whitening materials are categorized as professionally administered in-office treatments, professionally dispensed at-home treatments, or self-administered over-the-counter (OTC) whitening products.2 Many clinicians recommend in-office whitening to ensure patients receive professional guidance, gingival tissue protection, and more immediate results.3,4 However, the main difference between the three treatments is the amount of hydrogen peroxide present in the whitening material.
Used for more than a century, hydrogen peroxide (H2O2 or HP) is the most commonly used ingredient for teeth whitening.5 All tooth-whitening materials use HP and/or carbamide peroxide (urea peroxide or CP) as active ingredients.6-8 CP breaks down into HP and urea, and because of its slower breakdown process, the active agent is available in bleaching trays for more than 10 hours,9 making it ideal for whitening throughout the night. HP works best when used for 30 to 60 minutes because it becomes inactive quickly.10-12
Concentrations of HP vary among whitening materials; in-office whitening typically ranges from 25% to 40%, and at-home materials range from 3% to 9% HP. Some at-home whitening materials can contain up to 15%.1 However, the greater the concentration and contact time, the greater incidence of tooth sensitivity.13
Tooth sensitivity is the most common side effect associated with, and greatest deterrent for patients to successfully complete, whitening treatments.14,15 Tooth sensitivity due to temperature changes occurs in up to 50% of subjects following whitening.16 Gingival irritation is also commonly observed after bleaching, and when using the tray system, poor-fitting trays are typically the cause for any irritation.1 Although many patients cannot differentiate tooth sensitivity from gingival irritation,17-20 gingival irritation is typically not the limiting factor or a barrier for completing the treatment.1 Topical gels are now available to reduce tooth sensitivity and provide patient relief. Additionally, many at-home OTC bleaching products include additives (eg, potassium nitrate) to reduce sensitivity and gingival irritation.21
The use of light to accelerate the whitening process has been somewhat controversial.22-24 Theoretically, the advantage of using a light source is to heat the HP, increasing the rate of oxygen decomposition and accelerating free radical release, boosting the rupture of stain-containing molecules.22,23 Research has demonstrated that the use of light can improve whitening efficacy,25-27 and closer analysis of light-based whitening studies revealed that whitening gels with lower HP concentrations (15% to 25%) showed improved results with light.2,28 A light source activates the whitening process for lower-concentration HP gels by increasing the hydroxyl radicals,2 improving the degradation rate of hydrogen peroxide.29
Unlike in lower concentrations, when 35% HP is used, the benefits of light activation are not seen.27,30,31 It is hypothesized that the quantity of radicals already produced by HP degradation reaches the maximum amount necessary to react with the pigments in the dentin, and any increase in the radicals does not accelerate the whitening process.2
Additionally, Al Shethri et al determined that a single in-office whitening treatment does not elicit maximum whitening results.32 Therefore, a combination of in-office whitening with light source and take-home tray whitening may be necessary to achieve optimum whiteness for a patient. Although research is available regarding several aspects of the whitening process, the use of a combination technique (both in-office and at-home), as well as specific whitening gels (ie, Philips Zoom WhiteSpeed 25% HP, Philips Zoom NiteWhite 16% CP, Philips Oral Healthcare, www.philipsoralhealthcare.com) had not been investigated to verify a successfully combined professional whitening system until now. Philips Zoom products were utilized in this case study because they have consistently demonstrated effective whitening capabilities.25,33,34
Purpose
The primary objective of this case study was to assess via individual case analyses the efficacy of a combination professional in-office and take-home whitening protocol for discolored teeth between shades A3.5 and A4. A combination of currently available professional whitening products was tested to produce an esthetically appealing outcome while managing sensitivity. Two different whitening treatments were used to determine the whitening results achieved if follow-up at-home whitening trays were used as part of the treatment protocol. Additionally, two methods to reduce sensitivity were used to determine if sensitivity could be significantly reduced or eliminated from the whitening treatment.
Materials and Methods
Participants
Nine healthy volunteers were selected and enrolled as subjects for this study. Participants were required to be 16 to 80 years old and in generally good health. They all had a starting tooth shade of A3.5 or darker on the VITA Classical (VC) shade guide (Vident, www.vident.com) on four to six of their maxillary anterior teeth. (Note: The VITA Classical shade guide used to evaluate patients’ tooth shades in this study is shown above in the right hand column.) Participants were required to have four of the six maxillary anterior teeth non-restored and their teeth professionally cleaned during the past year. They were to be willing to abstain from use of whitening products not associated with the study, with the exception of toothpaste provided with the study, and willing to refrain from smoking and consuming dark-staining food and drink (coffee, colas, red wine, grape juice) for 2 hours after Philips Zoom WhiteSpeed whitening treatments and for 30 minutes after removing take-home whitening trays.
Participants were excluded for having restorations, crowns, veneers, or dental implants on more than two of the six maxillary anterior teeth as well as untreated caries, cracks, or large chips. Participants with severely malposed anterior teeth, visible supragingival calculus on the facial surfaces of the maxillary anterior teeth, or a history of systemic disease (which in the opinion of the examiner may have interfered with the assessment of oral hard and soft tissue) were also excluded. Participants with a known history of tetracycline use and/or the presence of tetracycline staining on teeth or dark brown fluorosis were not included. Those who had previously used professionally dispensed take-home or Philips Zoom WhiteSpeed whitening products or OTC whitening strips in the past 2 years were also excluded. Additional exclusions included subjects with known sensitivities to peroxides or glycols, subjects with current sensitivity on maxillary anterior teeth, pregnant or nursing subjects, or subjects that were unwilling to complete all study procedures.
Study Design
The 6-week case study required weekly subject visits for supplies, assessments, and whitening. Completed at 4 different clinical locations, evaluators followed the same protocol and utilized at least two dental professionals and a standard shade guide. Fluoride toothpaste with potassium nitrate (KNO3) (ie, Fluoridex, Philips Oral Healthcare) was used 1 week prior to the first whitening treatment and throughout the study period. This toothpaste was utilized to reduce the incidence of sensitivity throughout the treatment. For the baseline appointment and at each subsequent visit, clinical photographs were taken based on the American Academy of Cosmetic Dentistry’s “A Guide to Accreditation Photography” guidelines, including full face and 1:3 retracted views. To standardize documentation among evaluators, baseline shades were recorded using the VITA Classical shade guide, arranged in shade order, along with photographs of teeth Nos. 6 through 11.
For the whitening treatment, a liquid dam was placed to isolate the teeth and provide a gingival barrier. Philips Zoom WhiteSpeed (25% HP) whitening gel was then applied for four 15-minute sessions. Suction occurred between each session, followed by a final rinse after the fourth session. Light was used for these treatments because previous research indicated that light would enhance the 25% HP whitening gel effect.2,25-28 Custom trays were prepared for each individual subject to avoid gingival irritation and used at home to self-apply Philips Zoom NiteWhite (16% CP). Amorphous calcium phosphate (ie, Philips Relief ACP, Philips Oral Healthcare), a desensitizing agent, was recommended as needed to manage post-treatment sensitivity.
First and Second Visits
At the first visit, subjects were screened to ensure they met inclusion criteria. A dental prophylaxis was performed, including maxillary and mandibular anterior polish. Light lingual calculus was removed as necessary, with care to avoid traumatizing the gingival tissue. Take-home custom whitening trays were fabricated and Philips Fluoridex with potassium nitrate (KNO3) was given to subjects with instructions to be used for 1 week prior to the whitening treatment. Baseline photographs were taken and the subjects discharged.
During the second visit, the Philips Zoom WhiteSpeed treatment was performed on the maxillary anterior teeth (Nos. 6 through 11) as per the instructions, and Relief ACP was applied for 30 minutes following the treatment. Subjects were instructed to use the remaining relief gel as needed. Color assessment (VITA Classical) was performed on the whitened teeth and photographs taken to document shade change.
A 1-week supply of Philips Zoom NiteWhite (NW) was given to all study subjects to be used nightly for 1 week, starting 24 hours after the in-office treatment. Subjects were instructed to wear the tray overnight (every night) for a minimum of 4 to 6 hours.
Third, Fourth, and Fifth Visits
Study subjects returned for color evaluation of the maxillary teeth (shade assessment and photography repeated). Post-treatment soft-tissue and teeth sensitivity evaluations were performed, and if a B1 shade was not achieved, then an additional 1-week of NW was dispensed. Relief ACP was given for use as needed. After 2 weeks of NW use, if B1 was not achieved, then an additional Zoom WhiteSpeed treatment was offered to the subject.
Sixth Visit
The final post-whitening visit included the concluding assessment and photographs. If the subject’s teeth reached shade B1 before the sixth visit, results were documented and the treatment terminated for that particular subject.
Results
From the initial Philips Zoom WhiteSpeed treatment, subjects exhibited an average 9.56 shade changes on the VITA Classical guide set in value order. After 1 week of Philips Zoom NiteWhite treatment, all nine subjects demonstrated an average 10.32 shade changes on the same VITA Classical guide. One patient chose to exit the study 1-week post WhiteSpeed treatment due to sensitivity, although the patient was highly satisfied with the treatment results.
Upon completion of the established case study protocol, the nine subjects demonstrated an average change of 11.1 shades (Table 1). The patient with the least shade change still demonstrated an improvement of 5 shades, and the patient with the next lowest shade change demonstrated a change of 8.83 shades. Two subjects achieved a maximum change of 14 shades.
Case Studies
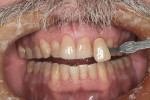
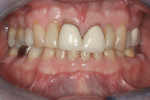
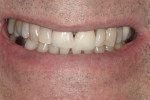
Subject #1—The patient was interviewed in the office and evaluated for the study. The six maxillary anterior teeth were determined to be A3.5 to A4 (Figure 1) through subjective shade selection and verified by the clinician and assistant using a digital shade-taking device (VITA EasyShade, Vident). Study protocol and informed consent were reviewed, and the patient agreed to proceed. The patient completed four 15-minute in-office treatment sessions of whitening per the case study protocol. Immediate results were noted; a shade of A2 to A3 was identified on the six maxillary anterior teeth, which was a change of 7 shades. The patient reported no sensitivity during the procedure.
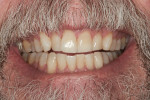
At-home protocol was followed, and the patient returned in 1 week for evaluation, at which time the patient reported no issues with sensitivity or a need to use the Philips ACP relief gel. The patient reported missing one or two nights of using Zoom NiteWhite, and evaluation (both subjective and objective) of shades demonstrated that the maxillary anterior teeth were between B2 and C2; a change of 9.66 shades. At visits 2 and 3 weeks post Zoom WhiteSpeed treatment, the subject continued to report no sensitivity, and shade changes became more subtle. The teeth that were at B2 maintained that shade, while teeth at shade C2 were a shade lighter at C1. At the 3-week appointment, the patient was offered one more Zoom WhiteSpeed in-office treatment but declined because he was very pleased with the whitening results (Figure 2).
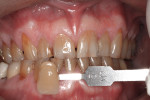
Subject #2—The patient was interviewed in the office and evaluated for the study. The six maxillary anterior teeth shades were determined using subjective shade selection and verified by the clinician and assistant as an A4 (Figure 3). After review of the study protocol and informed consent, the patient agreed to proceed. The patient completed the required in-office Philips Zoom WhiteSpeed service as per study protocol. Immediate results were noted; a shade change of A1 to B1 was noted on the six maxillary anterior teeth, which was a shade change of 13.33 shades. The patient reported no sensitivity during the procedure.
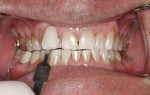
Following case study protocol, the patient continued nightly whitening treatments using NiteWhite as instructed. Subtle but noticeable shade changes were achieved. The central incisors that were B1 after in-office whitening maintained their B1 shade, but the lateral incisors made a minor shift from shade A1 to B1. The canines maintained an A1 shade throughout the take-home procedure. The patient was happy with the final results (Figure 4) and declined a second Zoom WhiteSpeed treatment.
Subject #9—The patient was interviewed in the office and evaluated for the study. The patient had 10+-year-old porcelain-fused-to-metal (PFM) crowns on his centrals, Nos. 8 and 9, both exhibiting shade B1 VITA (Figure 5). The lateral incisors and canines were documented as shade A4. After review of the study protocol and the informed consent, the patient completed the required in-office Philips Zoom WhiteSpeed procedure as per the protocol. The results demonstrated the natural teeth were significantly closer in shade to the existing crowns. The subject then completed the full Zoom NiteWhite protocol, achieving 14 shade changes and reaching B1 VITA after NiteWhite therapy. The patient was happy with the final results (Figure 6) and declined a second Zoom WhiteSpeed treatment.
Discussion
The results of the case study on nine subjects in four distinctly different geographical clinical locations established that Zoom WhiteSpeed in combination with NiteWhite therapy provided dramatic improvement in subjects with dark yellow shaded (A3.5 to A4) teeth. Results demonstrated that the most vivid improvement was achieved with the initial in-office Philips Zoom WhiteSpeed treatment, averaging 9.56 shade changes overall. However, with continued use of the at-home NiteWhite treatments, further improvements were consistently observed 1 to 3 additional shade changes.
Only two subjects (#3 and #6) elected to receive the optional Zoom WhiteSpeed treatment performed after 3 weeks of NiteWhite therapy. Both experienced further improvement; one subject (#6) was the least responsive prior to the second WhiteSpeed treatment. This suggests that further study with a larger population may be of interest to determine another combination protocol when subjects demonstrate slow progress or are resistant to whitening therapy.
A majority of subjects saw dramatic improvement, and when offered a second in-office WhiteSpeed therapy, they declined, stating complete satisfaction with the results. One patient exited the study after experiencing sensitivity. The sensitivity did resolve within 48 hours, and the subject was pleased with the results, demonstrating an 11-shade improvement. Another subject reported minor sensitivity that responded quickly and completely to the Relief ACP protocol. The subject was able to finish the case study and showed excellent results. All other study subjects reported no sensitivity during treatments or throughout the study. This could be due to the use of Fluoridex with KNO3 and desensitizing gel prior to the initial WhiteSpeed treatment and throughout the study.
Conclusion
This case study evaluated the combination of in-office and take-home tooth-whitening treatments. At the completion of the study, final shade change compared to baseline documentation averaged 11.1 shades overall. Results of this case study based on combined treatment and materials suggest successful whitening systems are available to effectively achieve dramatic, safe, and comfortable whitening for patients with darkly stained yellow teeth.
DISCLOSURE
The author is a consultant for Philips Oral Healthcare.
ABOUT THE AUTHOR
Gary M. Radz, DDS
Associate Clinical Professor, Department of Restorative Dentistry, University of Colorado School of Dental Medicine, Aurora, Colorado; Private Practice, Denver, Colorado
REFERENCES
1. Li Y, Greenwall L. Safety issues of tooth whitening using peroxide-based materials. Br Dent J. 2013;215(1):29-34.
2. Kossatz S, Dalanhol AP, Cunha T, et al. Effect of light activation on tooth sensitivity after in-office whitening. Oper Dent. 2011;36(3):251-257.
3. Luk K, Tam L, Hubert M. Effect of light energy on peroxide tooth bleaching. J Am Dent Assoc. 2004;135(2):194-201.
4. Tay LY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc. 2009;140(10):1245-51.
5. Fitch CP. Etiology of the discoloration of teeth. The Dental Cosmos. 1861;3(3):133-136.
6. Li Y. Biological properties of peroxide-containing tooth whiteners. Food Chem Toxicol. 1996;34(9):887-904.
7. Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51(2):319-331.
8. Li Y. Safety controversies in tooth bleaching. Dent Clin North Am. 2011;55(2):255-63.
9. Matis BA, Gaiao U, Blackman D, et al. In vivo degradation of bleaching gel used in whitening teeth. J Am Dent Assoc. 1999;130(2):227-235.
10. Matis BA. Tray whitening: what the evidence shows. Compend Contin Educ Dent. 2003;24(4A):354-362.
11. Al-Qunaian TA, Matis BA, Cochran MA. In vivo kinetics of bleaching gel with three-percent hydrogen peroxide within the first hour. Oper Dent. 2003;28(3):236-341.
12. Hein DK, Ploeger BJ, Hartup JK, et al. In-office vital tooth bleaching–what do lights add? Compend Contin Educ Dent. 2003;24(4A):340-352.
13. Leonard RH Jr, Garland GE, Eagle JC, Caplan DJ. Safety issues when using a 16% carbamide peroxide whitening solution. J Esthet Restor Dent. 2002;14(6):358-367.
14. Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent. 2005;26(9 suppl 3):11-20.
15. Swift EJ Jr. Tooth sensitivity and whitening. Compend Contin Educ Dent. 2005;26(9 suppl 3):4-10.
16. Schulte JR, Morrissette DB, Gasior EJ, Czajewski MV. Clinical changes in the gingiva as a result of at-home bleaching. Compendium. 1993;14(11):1362,1364-1366.
17. Kugel G, Aboushala A, Zhou X, Gerlach, RW. Daily use of whitening strips on tetracycline-stained teeth: comparative results after 2 months. Compend Contin Educ Dent. 2002;23(1A):29-34.
18. Gerlach RW, Zhou X. Comparative clinical efficacy of two professional bleaching systems. Compend Contin Educ Dent. 2002;23(1A):35-41.
19. Gerlach RW, Sagel PA, Jeffers ME, Zhou X. Effect of peroxide concentration and brushing on whitening clinical response. Compend Contin Educ Dent. 2002;23(1A):16-21.
20. Demarco FF, Meireless SS, Masotti AS. Over-the-counter whitening agents: a concise review. Braz Oral Res. 2009;23 suppl 1:64-70.
21. Leonard RH Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital whitening. Quintessence Int. 1997;28(8):527-534.
22. Caviedes-Bucheli J, Ariza-García G, Restrepo-Méndez S, et al. The effect of tooth whitening on substance P expression in human dental pulp. J Endod. 2008;34(12):1462-1465.
23. Rosenstiel SF, Gegauff AG, Johnston WM. Duration of tooth color change after bleaching. J Am Dent Assoc. 1991;122(4):54-59.
24. He LB, Shao MY, Tan K, et al. The effects of light on whitening and tooth sensitivity during in-office vital whitening: a systematic review and meta-analysis. J Dent. 2012;40(8):644-653.
25. Ontiveros JC, Kugel G. In-office whitening treatment. Inside Dentistry. 2014;10(6):88-92.
26. Ontiveros JC, Paravina RD. Color change of vital teeth exposed to whitening performed with and without supplementary light. J Dent. 2009;37(11):840-847.
27. Papathanasiou A, Kastali S, Perry RD, Kugel G. Clinical evaluation of a 35% hydrogen peroxide in-office whitening system. Compend Contin Educ Dent. 2002;23(4):335-3348.
28. Radz GM. Enhancing the efficiency and efficacy of in-office whitening using a chairside lamp. Compend Contin Educ Dent. 2013;34(6):466-470.
29. Cooper JS, Bokmeyer TJ, Bowles WH. Penetration of the pulp chamber by carbamide peroxide whitening agents. J Endod. 1992;18(7):315-317.
30. Marson FC, Sensi LG, Vieira LC, Araujo E. Clinical evaluation of in-office dental bleaching treatments with and without the use of light-activation sources. Oper Dent. 2008;33(1):15-22.
31. Bernardon JK, Sartori N, Ballarin A, et al. Clinical performance of vital bleaching techniques. Oper Dent. 2010;35(1):3-10.
32. Al Shethri S, Matis BA, Cochran MA, et al. A clinical evaluation of two in-office bleaching products. Oper Dent. 2003;28(5):488-495.
33. Gallagher A, Maggio B, Bowman J, et al. Clinical study to compare two in-office (chairside) whitening systems. J Clin Dent. 2002; 13(6):219-224.
34. Ziemba SL, Felix H, MacDonald J, Ward M. Clinical evaluation of a novel dental whitening lamp and light-catalyzed peroxide gel. J Clin Dent. 2005;16(4):123-127.