Histopathologic Changes in Dental Pulp of Teeth with Chronic Periodontitis
Abstract:
OBJECTIVE: The aim of this study was to evaluate the histopathologic changes in dental pulp of teeth with chronic advanced periodontitis. METHODS: In 22 patients, 30 teeth were selected for inclusion. Patients had received no periodontal treatment. No teeth had caries, abrasion, attrition, erosion, trauma, or restoration. Radiographically, all teeth showed bone-support destruction to the apex. Thermal and cavity tests were used to evaluate pulp vitality. After tooth extractions, crowns were separated from roots at the cementoenamel junction. Both the crowns and the roots were prepared for histopathologic analyses. Radicular pulp was analyzed considering both coronal and apical halves. RESULTS: In 100% of the cases, coronal pulp exhibited soft connective tissue. In the coronal half of radicular pulp, soft connective tissue was present in 60% of the cases, fibrosis in 30%, and fibrosis associated with dystrophic calcification in 10%. In the apical half of radicular pulp, 6.6% of the cases demonstrated fibrosis; 23% exhibited fibrosis associated with pulp atrophy and secondary dentin; and 63.3% showed dfibrosis, pulp atrophy, secondary dentin, and diffuse calcification. CONCLUSION: Radicular pulp of teeth with chronic periodontitis presents characteristics compatible with pulp changes resulting from pulp aging. In such cases, endodontic treatment is not indicated to enhance periodontal treatment results.
As the dental organ develops, anatomic pathways that connect the endodontium and periodontium, such as the apical foramen, lateral and accessory canals, and permeable areas of dentin and root cement, are established.1-3 Therefore, a pulp pathology may be reflected through these pathways in the periodontium, or even aggravate a pre-existing periodontal problem.4,5
Although it is admissible for pulp pathology to present repercussions in the periodontium, the influence of periodontal destruction on the pulp status remains an issue of debate.6 Many researchers believe periodontal lesions cause changes of variable intensity in the pulp tissue7-12; however, other authors do not believe in these influences.13-15 In view of these controversies, the aim of this study was to analyze the possible histopathologic changes in the pulp of uniradicular teeth with chronic advanced periodontitis.
Materials and Methods
Study Population
A total of 22 patients (9 females and 13 males; ages 48 to 62 years) with chronic periodontitis were selected from the Federal Fluminense University School of Dentistry, Rio de Janeiro, Brazil. After ethical approval, all patients were verbally informed about the study and signed free and informed written consent for study participation. Each patient was systemically healthy and was not taking any medication.
The samples, all of which were indicated for extraction, included 30 single-rooted teeth with no caries, abrasion, attrition, erosion, or restoration. All selected teeth presented chronic periodontitis extended to the root apex. None of the patients had received previous periodontal treatment. The clinical probing depths and measurements of the distances from the cementoenamel junction (CEJ) to the gingival margin were obtained. Radiographically, all teeth showed support-bone destruction to the apex. These data were compared with the mean lengths of the tooth roots16 confirming the support tissues’ destruction to the apex. Thermal and cavity tests were used to evaluate pulp vitality.
Samples Preparation
After tooth extractions, the crowns were separated from the roots at the CEJ. Both the crowns and the roots were fixed with 10% neutral buffered formaldehyde for 7 days, decalcified with 7% ethylenedinitrilotetraacetic acid (EDTA) solution, and routinely processed for light microscopy. Serial sections 5µ in thickness were tested with Hematoxylin and Eosin and Gomori’s trichrome stains. Histologically, the radicular pulp of each tooth was submitted for histologic analysis, considering both the coronal and radicular halves of the root.
Results
Histologic Evaluation
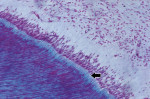
In the coronal portion, 100% of the samples presented loose connective tissue, with predominance of fibroblasts and few collagen fibers. The odontoblast layer at the periphery of the pulp connective tissue presented a palisade arrangement. The odontoblasts were separated from the mineralized dentin by predentin, which was shown as a layer of nonmineralized organic matrix indicative of odontoblastic activity (Figure 1), compatible with health.
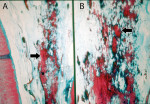
In the radicular pulp, the histologic findings included: 1) pulp atrophy, characterized by the reduction in pulp space due to the formation of reparative dentin, with reduction in the number and size of cells (Figure 2); 2) reparative dentin, very irregular with little canalicular disposition; 3) an increase in the number and thickness of collagen fibers (fibrosis), disposed in a thick web oriented in the axial direction of the canal (Figure 2); 4) dystrophic calcification, observed along the entire extension of the canal (These findings were presented in the form of irregular and elongated amorphous structures, disposed along the rich network of collagen fiber bundles.) (Figure 3 and Figure 4); and 5) inflammatory infiltrate, which was present in the connective pulp in the apical region with predominance of chronic inflammatory cells.
The results showed that in 100% of the cases the coronal pulp exhibited soft connective tissue resembling normal conditions. In the coronal half of the radicular pulp, soft connective tissue was present in 60% of the cases, fibrosis in 30%, and fibrosis associated with dystrophic calcification in 10%. In the apical half of the radicular pulp, 6.6% of the cases demonstrated fibrosis, 23% showed fibrosis associated with pulp atrophy and secondary dentin, and 63.3% exhibited fibrosis, pulp atrophy, secondary dentin, and diffuse calcification.
Discussion
The purpose of this study was to evaluate the histopathologic changes in dental pulp of teeth with chronic periodontitis. Although all teeth presented with bone-support destruction to the apex, they were vital and showed no caries, abrasion, attrition, erosion, trauma, restoration, or previous periodontal treatment.
The literature affirms that with pulp aging, the number of cells decreases and the fibrous components increase.17-21 In the present study, the incidence of fibrosis in the coronal and apical halves of radicular pulp was 40% and 100%, respectively. In the apical half of the radicular pulp, in all cases, fibrosis associated with reparative dentin, pulp atrophy, and dystrophic calcification was observed. The increase in fibrosis with the passage of time is not due to the continuous formation of collagen but could be due to the persistence of conjunctive fiber bundles in an increasingly narrow pulp cavity as a result of calcified tissue deposition.
A reduction in the number of blood vessels and nerves that support aging pulp was observed as early as 1975.17 This process was described as various arteries presenting signs of atherosclerosis similar to those observed elsewhere in the body. Another important finding is the presence of calcification in the arterioles and precapillary vessels, which was a frequent occurrence in the present study. Pulp calcifications, with varied shapes and sizes, accompanied by collagen fibers forming nodular structures, were also observed in the radicular pulp of the present study. These findings are compatible with the degenerative changes in pulp as a result of pulp aging, which is well documented in the literature.17-19,21 These changes may be found in the face of external aggressors, such as caries, abrasion, attrition, erosion, restorative procedures, periodontal disease, and periodontal treatment.7 In the present study, although periodontal disease had reached the root apex, causing pulp degenerative alterations, the indication of endodontic treatment was not justified. A reduction in pulp volume and increase in thickness of the dentin layer in teeth presenting with moderate to advanced chronic periodontitis was also found in another recent study.12
When studying pulp changes in caries-free or restored teeth with various degrees of periodontal disease, the occurrence of pulp calcifications associated with pulpitis and/or pulp necrosis as a result of inflammatory periodontal disease has been reported.7,9,22 No pulp necrosis was observed in the specimens in the present study. In addition, important parameters such as advanced periodontal involvement and absence of previous periodontal treatment were considered in the selection of the authors’ samples; these parameters were absent in previous studies.
In only two of the 30 pulps examined in the present study, inflammatory infiltrate was observed in the radicular pulp close to the apex. Langeland et al23 reported that various degrees of pulp inflammation were observed when bacterial plaque surrounded the root apex. Conversely, Badillo and Brouillet24 observed evidence of essentially degenerative pulp changes in all the teeth and extremely rare areas of chronic inflammation. Although the analysis of the results was not clear with reference to the degree of periodontal involvement, these findings are very similar to those found in the present study and suggest that periodontal disease causes acceleration of the degenerative process in the pulp organ.
The classic study by Czarneck and Schilder13 affirmed no correlation between the severity of periodontal disease and the presence or absence of pulp pathology. Notably, in that study, only the teeth with chronic periodontitis associated with caries and/or restorations showed inflammation and pulp necrosis. Teeth with periodontal disease that were free of caries and/or restorations presented pulps in the coronal portion within normal limits. These latter findings are compatible with those of the present study.
In recent research11 that used a model similar to the one used in the present study, Fatemi et al reported that inflammation was a common finding, with only 6.3% of teeth showing no inflammation. In addition, 64.6% of the pulps showed partial or complete necrosis. Pulp stones were absent in 77.1% of the samples. Conversely, in the present study, only 6% of the radicular pulps showed inflammation, and no necrosis was observed. Moreover, in the coronal half of the radicular pulp, calcification was present in 10%, whereas, in the apical half of the radicular pulp, 63.3% showed diffuse calcification. Similar to the Fatemi study, the results from the present study showed presence of fibrosis in more than 90% of the samples. While the Fatemi study concluded that moderate to severe chronic periodontitis can affect dental pulp, this statement conflicts with the conclusion of the present study and should be carefully evaluated, because a wrong treatment decision can sacrifice endodontically healthy teeth.
In regard to the coronal portion of the pulp, the present study showed loose connective tissue, with normal characteristics. This finding is compatible with studies that used caries-free teeth.13,22 It is important to note that the thickness of the predentin layer suggests odontoblastic activity, which is an indication that the connective tissue in the coronal portion remains vital and with fully functional metabolism.25
In the present study, it was not possible to differentiate between physiologic aging of the pulp and pulp aging due to periodontal disease. Ideally, the control and test teeth should be extracted from the same patient. However, this would require extraction of both a healthy tooth and a tooth condemned because of advanced periodontitis, which would be an extremely rare occurrence. Therefore, controlled studies have largely been confined to animal models.
Conclusions and Clincial Implications
Endodontic lesions are, in fact, expressed via the periodontium. However, the authors concluded that in the presence of periodontal lesions, it is of the ultmost importance to evaluate the pulp status. In the absence of inflammation and/or pulp necrosis, endodontic treatment will make no contribution to improving periodontal health.
The present study showed that radicular pulp of teeth with chronic advanced periodontitis presents characteristics compatible with pulp changes resulting from pulp aging. In such cases, endodontic treatment is not indicated to enhance periodontal treatment results.
References
1. Adriaens PA, De Boever JA, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally deseased teeth in humans. A reservoir of periodontopathic bacteria. J Periodontol. 1988;59(4):222-230.
2. Adriaens PA, Edwards CA, De Boever JA, Loesche, WJ. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59(8):493-503.
3. De Deus QD. Frequency, location, and direction of the lateral, secondary, and acessory canals. J Endod. 1975;1(11):361-366.
4. Hiatt WH. Histologic and clinical assessment of long-term pulpal-periodontal therapy. Oral Surg Oral Med Oral Pathol. 1982;54(4):436-440.
5. Jansson L, Ehnevid H, Lindskog S, Blomlöf FL. Relationship between periapical and periodontal status. A clinical retrospective study. J Clin Periodontol. 1993;20(2):117-123.
6. Mirot F, Dersot JM. Consequences of periodontal diseases and periodontal treatment in the pulp [in French]. J Parodontol. 1988;7(2):139-143.
7. Bender IB, Seltzer S. The effect of periodontal disease on the pulp. Oral Surg Oral Med Oral Path. 1972;33(3):458-474.
8. Rubach WC, Mitchell, DF. Periodontal disease, accessory canals and pulp pathosis. J Periodontol. 1965;36:34-38.
9. Rubach WC, Mitchell DF. Periodontal disease, age and pulp status. Oral Surg Oral Med Oral Pathol. 1965;19:482-493.
10. Serene TP. Interrelationship between endodontics and periodontics. J Ga Dent Assoc. 1967;40(4):14-17.
11. Fatemi K, Disfani R, Zare R, et al. Influence of moderate to severe chronic periodontitis on dental pulp. J Indian Soc Periodontol. 2012;16(4):558-561.
12. Heidari Z, Saberi EA, Mahmoudzadeh-Sagheb H, et al. Stereological analysis of the dental pulp in patients with advanced periodontitis. Zahedan J Res Med Sci. 2013;15(7):44-49.
13. Czarneck R, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. J Endod. 1979;5(8):242-253.
14. Mazur B, Massler M. Influence of periodontal disease of the dental pulp. Oral Surg Oral Med Oral Pathol. 1964;17:592-603.
15. Seltzer S, Bender IB, Nazimov H, Sinai I. Pulpitis-induced interradicular periodontal changes in experimental animals. J Periodontol. 1967;38(2):124-129.
16. Ash M, Nelson SJ, eds. Wheeler’s Dental Anatomy, Physiology, and Occlusion. 8th ed. St. Louis, MO: Saunders Elsevier; 2003:445.
17. Bernick S, Nedelman C. Effects of aging on the human pulp. J Endod. 1975;1(3):88-94.
18. Ketterl W. Age-induced changes in teeth and their attachment apparatus. Int Dent J. 1983;33(3):262-271.
19. Badillo F, Brouillet JL. Aging of the pulp dentin complex [in French]. Revue Française d’ endod. 1991;10(3):41-54.
20. Morse DR. Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg Oral Med Oral Pathol. 1991;72(6):721-745.
21. Ricci C. Aging of the pulp dentin complex: literature review and diagnostics conclusions [in French]. Revue Française d’endod. 1987;6(1):69-80.
22. Seltzer S, Bender IB, Ziontz M. The interrelationship of pulp and periodontal disease. Oral Surg Oral Med Oral Pathol. 1963;16(12):1474-1490.
23. Langeland K, Rodrigues HA, Dowden W. Periodontal disease, bacteria, and pulp histopatology. Oral Surg Oral Med Oral Pathol. 1974;37(2):257-270.
24. Badillo F, Brouillet JL. Histological aspects of pulp lesions in periodontal diseased teeth [in French]. J Parodontol. 1988;7(2):145-53.
25. Ten Cate AR. Oral Histology: Development, Structure, and Function. 4th ed. St. Louis, IL: Mosby; 1994:58-80.
About the Authors
Telma R. Aguiar, DScD
Associate Professor, Department of Periodontology, School of Dentistry, Fluminense Federal University, Rio de Janeiro, Brazil
Gilson C. Tristão, DScD
Associate Professor, Department of Prosthodontics, School of Dentistry, Fluminense Federal University, Rio de Janeiro, Brazil
Denize Mandarino, MScD
Associate Professor, Department of Periodontology, School of Dentistry, Fluminense Federal University, Rio de Janeiro, Brazil
Laila Zarranz, MScD
Assistant Professor, Department of Prosthodontics, School of Dentistry, Fluminense Federal University, Rio de Janeiro, Brazil
Vinícius F. Ferreira, MScD
Assistant Professor, Brazilian Institute of Periodontology, Rio de Janeiro, Brazil
Eliane P. Barboza, DScD
Associate Professor of Periodontology, School of Dentistry, Fluminense Federal University, Rio de Janeiro, Brazil