Down Syndrome-Associated Periodontitis: A Critical Review of the Literature
Abstract
Down syndrome, or trisomy 21 (T21), was first described by John Langdon Down in 1866. Down identified the phenotypic expression of patients with circulation and coordination problems as having Down syndrome. More than a century later, Jerome Lejeune hypothesized that nondysjunction during meiosis could lead to trisomy of the 21st chromosome. The incidence of T21 is one in 800 to 1,000 live births in the United States. Generally, these patients now live to age 50 and some to age 60. As life expectancy increases, medical and social costs garner greater attention. Also, societal changes have allowed for better quality of life. Dental practitioners are challenged by the high incidence of early onset aggressive periodontal disease in T21; these patients have higher levels of periodontal pathogens and periodontitis-associated interproximal bone loss. The complex anatomy, physiology, immunology, and microbiology underscore the need for further investigation in specific areas related to dental treatment of these patients. This article is a critical review of the periodontal research concerning T21. Creating awareness enables dental professionals who have the power and knowledge to appropriately address the needs of those affected by T21.
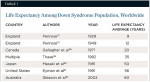
Trisomy of the 21st chromosome is present with phenotypic expression in 95% of patients with Down syndrome, or trisomy 21 (T21).1,2 The remaining cases are attributed to other chromosomal abnormalities, including translocation (3%), mosaicism (2%), and partial trisomy.2 The phenomenon occurs during spermatogenesis or oogenesis, resulting in a chromosome that has three copies. The incidence of T21 is one in 800 to 1,000 live births in the United States.1 Worldwide, life expectancy among this population is increasing (Table 1). The disorder has immunologic and physiologic differences that contribute to these patients’ poor periodontal condition.
A study by Cohen found severe periodontal disease affects 96% of individuals with T21,3 while others report this to be close to 100%.4 The high incidence of the disorder requires an understanding of physiologic, immunologic, anatomic, and microbiologic differences within this specific population in order to formulate the best treatment plan. Patients with T21 have various limitations that require consideration during diagnosis, treatment planning, and treatment. This article aims to critically review and highlight the collective research concerning these patients and underscore the need for further investigation in specific areas related to their dental treatment.
Physiologic and Anatomic Differences
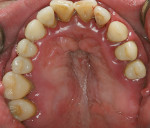
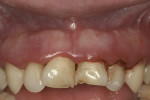
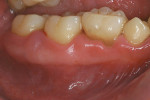
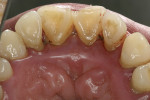
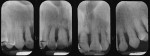
The extraoral appearance is distinct and consists of epicanthal folds at the eyelids, a broad nasal bridge, frontal bossing, open mouth, and an underdeveloped midface (Figure 1).5 Intraorally, patients exhibit macroglossia, high-arched palates (Figure 2), prognathism, and fissured tongues.3 Smaller permanent dentition6 and larger deciduous dentition7 are commonly seen. However, missing teeth are the most frequently observed dental anomaly in these patients.8 A higher incidence of dental abnormalities (Figure 3 through Figure 5), such as shovel-shaped incisors, missing or reduced marginal ridges, nippled appearance of canine tips, and wrinkled occlusal surfaces of molars, are believed to occur during tooth development.9
Patients with T21 have an increased incidence of gastrointestinal tract anomalies, congenital heart disease, immunodeficiency, nutritional difficulties during infancy, seizure disorders, sleep apnea, visual impairment, audiological dysfunction, weight gain toward adulthood, and thyroid disorders.10 The immunologic problem is believed to be the major constituent in the high mortality rate seen in these patients.11
Despite the anatomic differences, the larger concern in relation to periodontitis is with the altered physiology. The explanation for the advanced periodontal destruction may include the diminished chemotaxis of neutrophils, decreased phagocytic ability, and a suspected shortened half-life of the neutrophils.12 However, chemotaxis is the main kink in the cascade of the immune response.13-15 Neutrophils are responsible for the first line of defense against infection, and their absence sets the stage for disease.
Studies examining the polymorphonuclear leukocytes’ (PMNs) activity toward Aggregatibacter actinomycetemcomitans in patients with T21 compared to age-matched controls denote a significant decrease in activity.16 The advanced tissue destruction seen in the T21 population can, in part, be attributed to both endogenous and exogenous collagenase activity inductions. Exogenous activation, seen in matrix metalloproteinase-2, has been shown to be activated by A. actinomycetemcomitans.17 Zhou reported increased collagen degradation by human gingival fibroblasts coinciding with increased activation of MMPs and lowered tissue inhibitor of metalloproteinase protein (TIMP) levels when exposed to Porphyromonas gingivalis.18 In addition, levels of prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and matrix metallopeptidase-9 in gingival crevicular fluid (GCF) from patients with T21 were higher compared to matched controls.19
Halinen et al detected increased amounts and activity of neutrophil-derived matrix metallopeptidase-8 in patients with T21 compared to age-matched controls.20 Another strong link is found with juvenile periodontitis. The two groups have the same pattern of tissue destruction (Figure 6) and a problem with neutrophil chemotaxis. Lavine et al in 1979 found 86% of patients with juvenile periodontitis exhibited significant inhibition of PMN chemotaxis with no change in phagocytic capacity.21 Other arguments for the parallels between the two groups are based on their bacterial constitution and viral co-infections, which are explored in this article.
Clinical Appearance
The clinical and radiographic appearance of periodontitis in the patient with T21 often resembles the pattern of bone loss observed in localized juvenile periodontitis (LJP). A study by Modéer found that the first signs of periodontitis may be seen as early as 11 years of age, with most lesions detected in the mandibular incisors.22 Other affected teeth include the maxillary and mandibular first molars; canines are the least affected. In general, patients with T21 are known to have a higher plaque index and increased tooth mobility.23,24 Cutress found patients with T21 presented with more bone loss compared with other individuals who had intellectual limitations in the same institution. Others have reported patients with T21 have poor circulation and hormonal disturbances.25
Etiology
Oral hygiene is often used as a predictor of patients’ caries experiences. Poor oral hygiene directly correlates to the degree of mental retardation as do increased rates of caries among those populations. Limited access to care, limited manual dexterity, and lowered efficacy of self home care are all factors in the increased levels of gingivitis in those who have mental disabilities compared to controls.26 A possible conclusion is that the same should be true for the patient with T21; however, dental caries are less prevalent.27,28 Studies of aggressive periodontitis have also shown an absence of caries despite high plaque levels, suggesting a correlation between aggressive periodontitis and T21.29,30
Microbiologic Findings
With the poor oral hygiene exhibited by patients with T21, the potential for a robust flora arises. Comparisons of patients with mental challenges and those with T21 have demonstrated individuals with T21 had higher amounts of periodontal pathogens in addition to greater amounts of interproximal bone loss and periodontitis.31 Cichon found high levels of P. gingivalis, motile organisms, and spirochetes in patients with T21 compared to individuals with cerebral palsy.8 P. gingivalis, Tannerella forsythia, and A. actinomycetemcomitans were found in more than 30% of patients with T21.32 Some studies argue no specific effect of microbial composition in the destruction seen in patients with T21 when compared with matched patients with mental challenges.33 Other findings have shown no significant difference in levels of A. actinomycetemcomitans when comparing healthy patients with T21 and controls.34 However, the preponderance of evidence shows otherwise. Reports by Sakellari show A. actinomycetemcomitans in 35% of individuals with T21.35 A higher level of serum antibody to A. actinomycetemcomitans is found in these patients compared to controls.36 Higher immunoglobulin M antibody levels to A. actinomycetemcomitans and higher levels of immunoglobulin G antibodies to P. gingivalis are also found in patients with T21 and periodontitis.37 In addition, when compared to healthy controls, higher amounts of P. gingivalis are found in persons with T21.38
Bacteria have long been suspected as key elements in the destruction seen in periodontal disease. Recently, a cofactor in the entire constitution of periodontal destruction has been introduced with viruses. Contreras et al has observed that patients with aggressive periodontitis have viruses such as Epstein-Barr virus (EBV) type 1 and human cytomegalovirus (HCMV).39,40 The possibility of co-infection with herpes simplex virus (HSV) or members of that family along with periodontopathic bacteria has been offered as a reason for the amount of destruction in patients with T21.41 The presence of EBV and HCMV was found in deeper pockets of individuals with T21, further suggesting a relationship between the destructive capacity of the co-infection.42 Studies have suggested the presence of a herpes virus bacterial co-infection in the development of LJP, with higher levels of A. actinomycetemcomitans found with active HCMV lesions.42 This suggests a potential etiology of a co-infection by bacterial and viral entities, that may lead to the periodontal destruction seen in T21. This potential may also be extended to a type of tissue tropism, explaining the destruction seen in specific areas of the dentition. However, additional research is necessary to make any further claims.
Treatment
The family is an important component in the treatment. Because many of these patients lack the comprehension or manual capacity to perform dental hygiene, family members should be entrusted with this responsibility. Any oral hygiene instruction should be given to the family member or caretaker. Attempts at restoring the dentition are met with difficulty in the area of removable prosthesis and may be considered a contraindication.43 The need for anesthesia and sedation in surgical treatment of patients with T21 requires a careful and systematic review of the patient’s individual status. The respiratory system of these patients presents with a smaller tracheal tube for age-matched controls, high adenoidal hypertrophic incidence, macroglossia, a high- and narrow-arched palatal vault, and preoperative existence of respiratory tract infection.44 Cardiovascular challenges include a predisposition to pulmonary hypertension stemming from a left to right shunt.44 Anatomic differences in T21 are often seen in atlantoaxial instability (AAI). This has been reported to cause spinal cord injuries from patient positioning during surgery that involves the use of general anesthesia.45 Patients exhibiting symptoms or those who have received a diagnosis of AAI may require special handling so as to avoid any neck flexion during head posturing.1
Scaling and root planing as a primary therapy should be followed with greater frequency and performed early in life. As with the other recall protocols, the intent is to monitor and slow disease. In regard to the similarities to LJP, especially the high incidence of A. actinomycetemcomitans and other periodontopathic bacteria, adjunctive antibiotic therapy should be considered. The use of singular antibiotics or antibiotics without mechanical therapy has failed to show efficacy.46 In one study, the combination of metronidazole and amoxicillin decreased A. actinomycetemcomitans in 16 out of 22 patients with LJP and rapidly progressive periodontitis (RPP) for 1 year.46 Metronidazole and amoxicillin in LJP, advanced periodontitis (AP), and RPP reduced probing depths and bleeding on probing and increased clinical attachment levels.47 The successful treatment of aggressive cases involving A. actinomycetemcomitans when combining scaling and root planing, amoxicillin, and metronidazole has proven to be effective.48-50
Despite treatment, some cases continue to show periodontial destruction. A study by Barr-Agholme et al followed patients with T21 for 7 years, measuring the progression of periodontal disease. The results showed an increase in the number of participants exhibiting bone loss from 35% to 74%, with most lesions presenting in the mandibular incisors.51 Recent attempts have been made to treat suspected co-infections of severe periodontitis with antivirals in order to achieve resolution where traditional therapy has failed.52
With this continued progression of destruction and the longer life expectancy in T21, implant therapy may be an option. In a case report by Lustig,8 implants were placed in a patient with T21 to serve as abutments for single-tooth fixed prosthesis. Two implants placed in the maxilla (4.1 mm x 13 mm) and two placed in the mandible (3.75 mm x 11 mm and 3.75 mm x 13 mm) were followed for 8 months before loading. Both mandibular implants were lost before the loading period; one at 6 months and the other at 8 months. The cause for failure could not be determined with certainty; however, the reported osteoporotic nature of bone in T21 was suspected. The maxillary implants were subsequently loaded and placed into function. This limited research makes it difficult to conclude whether implants are an option for these patients.
Societal Considerations
When reviewing the costs to society and families, much of the literature focuses on cost from a quantitative and qualitative perspective. Direct costs of medical, developmental, and special education services and the indirect costs of lost work and household productivity have come to an estimated lifetime of $1.8 million.53 Some estimates have found there to be 12 to 13 times greater medical costs from 0 to 4 years in T21 versus those without.54 Some have noted the need for a parent to leave work to provide constant care and supervision of the child.55 The impact of insurance coverage throughout all income levels helps families avoid financial problems and reduces the need to leave work. Yet medical insurance often does not include provisions for assistance outside of medical needs.
Lacking from many of the cost and benefit analyses is the more qualitative focus, in which the value of these people to society and themselves is measured. As the mental capacity ranges in T21, so does the capacity for self-sufficiency and interaction. In a study by Alderson et al, people with T21 were interviewed directly. Many lived in homes without aid or direct care. When living with other patients with T21, they performed daily routines and various responsibilities. Personal accounts showed a happier living experience when independence was afforded.56
Conclusion
Patients with T21 are living longer. The incidence of aggressive periodontal disease among T21 patients is high. Along with periodontal disease, the patient population has other medical conditions that limit treatment. Poor oral hygiene, neutrophil dysfunctions, specific bacteria, and possible viral co-infections contribute to disease severity. After proper diagnosis, treatment often can slow the disease process and possibly shift the oral flora from periodontal pathogens. The literature regarding T21 and rehabilitation with dental implants is lacking. The limited work to date has only shown partial success, but no conclusions can be drawn. Further research is necessary to determine if patients with T21 are true candidates for implant therapy.
References
1. Galley R. Medical management of the adult patient with Down syndrome. JAAPA. 2005;18(4):45-52.
2. Desai SS. Down syndrome: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endon. 1997;84(3):279-285.
3. Cohen MM, Winer RA, Schwartz S, Shklar G. Oral aspects of mongolism. I. Periodontal disease in mongolism. Oral Surg Oral Med Oral Pathol. 1961;14:92-107.
4. Reuland-Bosma W, van Dijk J. Periodontal disease in Down’s syndrome: a review. J Clin Periodontol. 1986;13(1):64-73.
5. Cichon P, Crawford L, Grimm WD. Early-onset periodontitis associated with Down’s syndrome—clinical interventional study. Ann Periodontol. 1998;3(1):370-380.
6. Townsend GC. Tooth size in children and young adults with trisomy 21 (Down) syndrome. Arch Oral Biol. 1983;28(2):159-166.
7. Townsend GC. Dental crown variants in children and young adults with Down syndrome. Acta Odontol Pediatr. 1986;7(2):35-39.
8. Lustig JP, Yanko R, Zilberman U. Use of dental implants in patients with Down syndrome: a case report. Spec Care Dentist. 2002;22(5):201-204.
9. Cohen MM, Blitzer FJ, Arvystas MG, Bonneau RH. Abnormalities of the permanent dentition in trisomy G. J Dent Res. 1970;49(6 suppl):1386-1393.
10. Pueschel SM. Clinical aspects of Down syndrome from infancy to adulthood. Am J Med Genet Suppl. 1990;7:52-56.
11. Costello C, Webber A. White cell function in Down’s syndrome. Clin Genet. 1976;9(6):603-605.
12. Mittwoh U. The polymorphonuclear lobe count in mongolism and its relation to the total leukocyte count. J Ment Defic Res. 1958;2:75-80.
13. Barkin RM, Weston WL, Humbert JR, Sunada K. Phagocytic function in Down syndrome—II. Bactericidal activity and phagocytosis. J Ment Defic Res. 1980;24(pt4):251-256.
14. Izumi Y, Sugiyama S, Shinozuka O, et al. Defective neutrophil chemotaxis in Down’s syndrome patients and its relationship to periodontal destruction. J Periodontol. 1989;60(5):238-242.
15. Yavuzyilmaz E, Ersoy F, Sanal O, et al. Neutrophil chemotaxis and periodontal status in Down’s syndrome patients. J Nihon Univ Sch Dent. 1993;35(2):91-95.
16. Sreedevi H, Munshi AK. Neutrophil chemotaxis in Down syndrome and normal children to Actinobacillus actinomycetemcomitans. J Clin Pediatr Dent. 1998;22(2):141-146.
17. Tiranathanagul S, Yongchaitrakul T, Pattamapun K, Pavasant P. Actinobacillus actinomycetemcomitans lipopolysaccharide activates matrix metalloporteinase-2 and increases receptor activator of nuclear factor-kappaB ligand expression in human periodontal ligament cells. J Periodontol. 2004;75(12):1647-1654.
18. Zhou J, Windsor LJ. Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metaloproteinases. J Periodontal Res. 2006;41(1):47-54.
19. Tsiligaradis G, Yucel-Lindberg T, Modéer T. Enhanced levels of prostaglandin E2, leukotriene B4, and matrix metalloproteinase-9 in gingival crevicular fluid from patients with Down syndrome. Acta Odontol Scand. 2003;61(3):154-158.
20. Halinen S, Sorsa T, Ding Y, et al. Characterization of matrix metalloproteinase (MMP-8 and -9) activities in the saliva and in gingival crevicular fluid of children with Down’s syndrome. J Periodontol. 1996;67(8):748-754.
21. Lavine WS, Maderazo EG, Stolman J, et al. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979;14(1):10-19.
22. Modéer T, Barr M, Dahllöf G. Periodontal disease in children with Down’s syndrome. Scand J Dent Res. 1990;98(3):228-234.
23. Kisling E, Krebs G. Periodontal conditions in adult patients with Mongolism (Down’s syndrome). Acta Odont Scand. 1963;21:391-405.
24. Shapiro S, Gedalia I, Hofman A, Miller M. Periodontal disease and blood citrate levels in patients with trisomy 21. J Dent Res. 1969;48(6):1231-1233.
25. Cutress TW. Periodontal disease and oral hygiene in trisomy 21. Arch Oral Biol. 1971;16(11):1345-1355.
26. López-Pérez R, Borges-Yáñez SA, Jiménez-García G, Maupomé G. Oral hygiene, gingivitis, and periodontitis in persons with Down syndrome. Spec Care Dentist. 2002;22(6):214-220.
27. Winer RA, Cohen MM. Dental caries in Mongolism. Dental Progr. 1962;2:217-219.
28. Johnson MP, Young MA, Gallios JA. Dental caries experience of mongoloid children. J Dent Child. 1960;27:292-294.
29. Siosen PB, Furgang D, Steinberg LM, Fine DH. Proximal caries in juvenile periodontitis patients. J Periodontol. 2000;71(5):710-716.
30. Fine DH, Goldberg D, Karol R. Caries levels in patients with juvenile periodontitis. J Periodontol. 1984;55(4):242-246.
31. Gabre P. Studies on oral health in mentally retarded adults. Swed Dent J Suppl. 2000;(142):1-48.
32. Sakellari D, Belibasakis G, Chadjipadelis T, et al. Supragingival and subgingival microbiota of adult patients with Down’s syndrome. Changes after periodontal treatment. Oral Microbiol Immunol. 2001;16(6):376-382.
33. Reuland-Bosma W, van der Reijden WA, van Winkelhoff AJ. Absence of specific subgingival microflora in adults with Down’s syndrome. J Clin Periodontol. 2001;28(11):1004-1009.
34. Amano A, Kishima T, Kimura S, et al. Periodontopathic bacteria in children with Down syndrome. J Periodonol. 2000;71(2):249-255.
35. Barr-Agholme M, Dahllöf G, Linder L, et al. Actinobacillus Actinomycetemcomitans, Capnocytophaga, and Porphyromonas gingivalis in subgingival plaque of adolescents with Down’s syndrome. Oral Microbiol Immunol. 1992;7(4):244-248.
36. Santos R, Shanfeld J, Casamassimo P. Serum antibody response to Actinobacillus Actinomycetemcomitans in Down’s syndrome. Spec Care Dentist. 1996;16(2):80-83.
37. Morinushi T, Lopatin DE, Van Poperin N. The relationship between gingivitis and the serum antibodies to the microbiota associated with periodontal disease in children with Down’s syndrome. J Periodontol. 1997;68(7):626-631.
38. Amano A, Kishima T, Akiyama S, et al. Relationship of periodontopathic bacteria with early-onset periodontitis in Down’s syndrome. J Periodontol. 2001;72(3):368-373.
39. Contreras A, Slots J. Mammalian viruses in human periodontitis. Oral Microbiol Immunol. 1996;11(6):381-386.
40. Contreras A, Slots J. Active cytomegalovirus infection in human periodontitis. Oral Microbiol Immunol. 1998;13(4):225-230.
41. Hanookai D, Nowzari H, Contreras A, et al. Herpesvirus and periodontopathic bacteria in Trisomy 21 periodontitis. J Periodontol. 2000;71(3):376-384.
42. Ting M, Contreras A, Slots J. Herpesvirus in localized juvenile periodontitis. J Periodontal Res. 2000;35(1):17-25.
43. Allwork JJ, Edwards IR, Welch IM. Ingestion of a Quadhelix appliance requiring surgical removal: a case report. J Orthod. 2007;34(3):154-157.
44. Mitchell V, Howard R, Facer E. Down’s syndrome and anesthesia. Paediatr Anaesth. 1995;5(6):379-384.
45. Butler M, Hayes B, Hathaway M, Begleiter ML. Specific genetic diseases at risk for sedation/anesthesia complications. Anesth Analg. 2000;91(4):837-855.
46. van Winkelhoff AJ, Rodenburg JP, Goené RJ, et al. Metronidazole plus amoxicillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. J Clin Periodontol. 1989;16(2):128-131.
47. van Winkelhoff AJ, Tijhof CJ, de Graaff J. Microbiological and clinical results of metronidazole plus amoxicillin therapy in Actinobacillus actinomycetemcomitans-associated periodontitis. J Periodontol. 1992;63(1):52-57.
48. Christersson L, van Winkelhoff AJ, Zambon JJ, et al. Systemic antibiotic combination therapy in recalcitrant and recurrent localized juvenile periodontitis. J Dent Res. 1989;68:197.
49. Kornman KS, Newman MG, Flemming T, et al. Treatment of refractory periodontitis with metronidazole plus amoxicillin or Augmentin. J Dent Res. 1989;68:917.
50. Pavicić MJ, van Winkelhoff AJ, Douqué NH, et al. Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation. J Clin Periodontol. 1994;21(2):107-112.
51. Agholme MB, Dahllöf G, Modéer T. Changes of periodontal status in patients with Down syndrome during a 7-year period. Eur J Oral Sci. 1999;107(2):82-88.
52. Sunde PT, Olsen I, Enersen M, Grinde B. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J Clin Virol. 2008;42(2):176-178.
53. Waitzman NJ, Romano PS. Economic costs of birth defects and cerebral palsy—United States, 1992. MMWR Morb Mortal Wkly Rep. 1995;44(37):694-699.
54. Boulet SL, Molinari NA, Grosse SD, et al. Health care expenditures for infants and young children with Down syndrome in a privately insured population. J Pediatr. 2008;153(2):241-246.
55. Chen AY, Newacheck PW. Insurance coverage and financial burden for families of children with special health care needs. Ambul Pediatr. 2006;6(4):204-209.
56. Alderson P. Down’s syndrome: cost, quality and value of life. Soc Sci Med. 2001;53(5):627-638.
57. Penrose LS. The incidence of mongolism in the general population. J Ment Sci. 1949;95(400):685-688.
58. Gallagher RP, Lowry RB. Longevity in Down’s syndrome in British Columbia. J Ment Defic Res. 1975;19(3-4):157-163.
59. Thase ME. Longevity and mortality in Down’s syndrome. J Ment Defic Res. 1982;26(pt 3):177-192.
60. Masaki M, Higurashi M, Iijima K, et al. Mortality and survival for Down syndrome in Japan. Am J Hum Genet. 1981;33(4):629-639.
61. Eyman RK, Call TL. Life expectancy of persons with Down syndrome. Am J Ment Retard. 1991;95(6):603-612.
62. Glasson EJ, Sullivan SG, Hussain R, et al. The changing survival profile of people with Down’s syndrome: implications for genetic counseling. Clin Genet. 2002;62(5):390-393.
Related Content: Learn more about perio/pharma products at dentalaegis.com/go/cced131
About the Authors
Alon Frydman, DDS
Clinical Assistant Professo
Advanced Periodontology
Herman Ostrow School of Dentistry
University of Southern California
Los Angeles, California
Diplomat
American Board of Periodontology
Hessam Nowzari, DDS, PhD
Director
Advanced Periodontology
Herman Ostrow School of Dentistr
University of Southern California
Los Angeles, California
Diplomate
American Board of Periodontology