Titanium: The Future is Here
With the correct tools, this highly biocompatible material can benefit the laboratory, the dentist, and the patient.
When a crack forms between the connectors of a fixed zirconia, full-contour, four-unit bridge that is more than a year old, the dentist and technician know that something out of the ordinary has happened. In this case, the patient exhibited a heavy bite and an allergy to metal, which necessitated that the bridge be created using full zirconia with no porcelain overlay. The preparation design was excellent, but the bridge simply could not withstand the constant masticatory pressures.
The clinician was inclined to replace the bridge with yellow precious metal in order to completely avoid this problem in the future. However, with the size of the large pontic, the cost of the metal would be, without question, prohibitive. It seemed prudent to come up with an alternative solution.
Dental professionals have all encountered similar complications. This article will explore finding and creating a solution that is inexpensive and sustainable for the future. At this writing, an ounce of gold costs more than $1,400 on the London market. If technicians order an ounce of precious metal that contains more than 86% gold, they must pay more than $1,400 for it. Divide that by 20 for a single pennyweight (dwt) per crown, laboratory owners are paying $70 per unit without mark-up. For a single unit, this cost might be acceptable, but for a long-span bridge with a large pontic, the cost is driven up to approximately $500 to $700 for yellow metal, assuming the laboratory uses about a half-ounce of metal to create the restoration. Is there a solution to these skyrocketing costs?
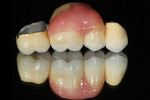
Having extensively experimented with titanium recently, the author was certain that this highly biocompatible material might be a viable option for this patient’s case. The cost is much lower and, at a flat rate, completely predictable. Titanium is approved by the American Dental Association and has been assigned code number 2794 for insurance purposes as a porcelain-to-titanium crown. The main problem the author has encountered is convincing clients that titanium is a durable material. Dentists are frequently worried about porcelain cracking and de-bonding from the substructure. The author decided to create the bridge for this patient using titanium instead of precious metal to see if the results were esthetically pleasing and functionally acceptable to the patient and clinician.
Case Presentation
The author scanned the master model using the NobelProcera™ scanner (Nobel Biocare, www.nobelbiocare.com) and CAD designed the four-unit Ti6A14V titanium bridge (Figure 1). After the scan design was transmitted to the Nobel Biocare facility for milling, the framework would be received back at the laboratory within 2 days. By using the NobelProcera scanner, the frame design can be controlled and delivered without the need for a wax-up or investing and divesting. The author’s concern prior to doing research on this framework material and construction method was that a CAD/CAM-designed and -milled titanium bridge would rock. However, this has not proved to be the case and fit has been consistently solid.
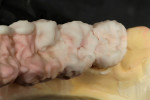
When the framework was delivered to the laboratory, the author ground the copings using a KOMET carbide bur (www.kometusa.com) (Figure 2). The author took great care to grind the copings in one direction only to ensure a smooth finish to prevent porcelain bubbling. The carbide instruments make grinding effortless, with exceptional results (Figure 3). The framework was next sandblasted using a Renfert sandblaster (www.renfert.com) at two to four bars of pressure at 50 µm. Figure 4 and Figure 5 shows before and after views of the author’s sandblasting efforts and the appearance of the titanium framework.
Before overlaying a titanium framework with porcelain, an initial layer of bonder must be applied. This is one of the most important steps to take into account when working with titanium. Do not de-gas the framework before applying the bonder. Titanium is a progressive oxide that appears dark after every firing. De-gassing will add another black layer to the surface. The bonder’s importance in creating these restorations cannot be overestimated. The author has had excellent results with GC Initial Ti Bonder (www.gcamerica.com). The bonder is applied in a very thin layer to the framework and baked at 810°C to protect against porcelain and opaque bubbling (Figure 6). Ti-Bonder’s main purpose is to create chemical bonding. Without this step, the porcelain cannot bond to titanium metal.
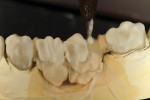
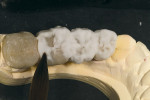
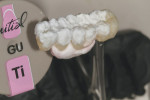
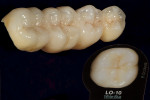
GC Initial Titanium Opaque (O-A4) porcelain was next applied to the framework (Figure 7). Figure 8 shows the framework after the first bake. Four GC Initial Titanium porcelain powder colors (Figure 9) show the wide range of shade possibilities available for titanium frameworks. The dentin layer plus translucency modifier were applied to the titanium understructure (Figure 10). Figure 11 demonstrates its color appearance before the second bake. After the second bake, a layer of enamel modifiers (EO-17 and E-58) were applied (Figure 12) and then a layer of GC Gum (Figure 13). The bridge was then ready for its third bake (Figure 14).
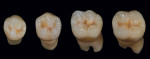
To study the different aspects and details of tooth morphology, such as the occlusal table, cusp ridge direction, secondary ridge and dissection groove, as well as the fosse, the author painted gold powder from Renfert onto the natural teeth shown in Figure 15. The continual study of the harmony of natural teeth helps the author when creating restorations such as the titanium bridge in this case study. From root to completion, the author also fabricated porcelain mandibular crowns, trying to mimic the natural tooth aspect (Figure 16). In order to correctly match the occlusal enamel and stain, the author used his proprietary Chairside Shade Guide (Figure 17) as his tool and then applied pink porcelain to the gum area (Figure 18) for an optimally natural appearance. Using the proper tools is paramount as a guide for re-creating the esthetics and function of natural teeth. To quote a very good friend of the author’s: "Always know your destination before you begin your journey." This is true in good dentistry as well. Research, development, and hard work will pay off by providing an effective base for the work of the future.
Conclusion
When working with titanium, in the author’s experience, we will all have the best luck if we follow the porcelain instructions carefully, keep our working area very clean, and do not combine the titanium porcelain with any other type. In fact, it is best to have a special section of the laboratory strictly for titanium work, with a separate oven and tools, as well. Because titanium has such specific properties that must be taken into consideration, any contamination can lead to inconsistent results.
As mentioned in the introduction, everyone worries about bubbling and cracking when using titanium. To avoid this, the heat rate at which these restorations are baked must be controlled. The metal CTE rate for is higher than that of porcelain, which means that the heat rate and hold time must be increased.
About the Author
Luke Kahng, CDT
Owner, LSK121
Naperville, Illinois