TheraCal™ LC
Achieve predictable and esthetic posterior restorations with a highly adaptive calcium silicate.
Protecting the pulp is the utmost responsibility of the clinician. “Seal to heal” is the goal of effective dentin protection. It is an accepted principle in restorative dentistry that partial caries removal in asymptomatic teeth is a standard we should consider.1,2 Adequate sealing of affected dentin aids in the naturally occurring dentin/pulpal healing response. Systematic reviews conclude that the removal of mainly infected dentin results in the following3:
- Significantly reduced iatrogenic pulpal exposures compared to complete caries removal.
- Evidential support for the principle of leaving affected dentin within sealed borders.
- The ability of affected dentin to be revitalized and serve as a scaffold from which to build protective dentin.
The reactive process of demineralization/remineralization in moderate to deep dentin is a dynamic one and represents the want/need of the tooth to heal itself. The reactive process is facilitated by many naturally occurring mechanisms within the tooth substrate but hindered by the inability of many commonly used dentin-lining agents to create a long-lasting seal and to promote apatite formation.3
Demineralized dentin represents a “wound” that the tooth naturally wants to heal. It is understood that to facilitate the tooth’s natural reactive healing process requires the following:
- Lining materials that are self-sealing without the aid of adhesives to prevent bacterial invasion.
- Adequate physical properties to withstand compressive forces under amalgam and composite.
- Minimal solubility to allow for etching and bonding during completion of the restoration.
- Radiopacity for visualization.
- Rapid, sustained calcium release to mobilize naturally occurring dentin matrix proteins resulting in apatite re-formation within affected areas.
- Rapid, sustained calcium release to mobilize naturally occurring dentin matrix proteins resulting in apatite reformation within affected areas.
Bonding directly with adhesives over exposed pulps has been shown to result in significant inflammatory responses.4,5 Resin-modified glass ionomer (RMGI) and calcium hydroxide are, to date, the most popular materials used for this purpose but separately, they lack many of the pulpal protecting properties required to promote adequate healing potential. Calcium hydroxide is very soluble, it has little natural bond to dentin, it lacks compressive strength, and it is susceptible to etching. RMGI is required to complete the procedure by providing a compressive barrier for the calcium hydroxide and seal from bacterial invasion.
RMGI alone has been shown to be cytotoxic and not perfectly indicated for use in deep dentin (interconnected to the pulp) or directly over exposed pulp.3 Poly-acrylic acid, present in all glass ionomers and un-reacted HEMA in RMGIs, has been shown to inhibit apatite formation in the body environment and to be cytotoxic to pulpal tissues.6,7 Calcium hydroxide as well as light-curable calcium hydroxide has been shown to produce very thin dentinal bridging.8
This improved knowledge was the basis for the introduction several years ago of mineral trioxide aggregate (MTA) in the form of ProRoot® MTA (DENTSPLY Tulsa, www.tulsadental.com) and, recently, Biodentine™ (Septodont, www.septodontusa.com). Both contain the apatite-stimulating calcium silicates that have been shown over many years to provide improved benefits of dentin stimulation and pulpal protection as compared to traditional calcium hydroxide and RMGI alone. Unfortunately, the cost per use, the slow setting qualities, and solubility hinder the use of these materials in everyday direct restorative dentistry.
A New Class of Interactive Flowable Materials
TheraCal™ LC (BISCO, www.bisco.com) is dentistry’s first radiopaque, HEMA-free, light-curable flowable resin containing “apatite stimulating” calcium silicates. It is the first of a new class of internal flowable materials to serve in protecting and stimulating pulpal repair and will likely be described by the profession as a light-curable resin-modified calcium silicate (RMCS).
Research supports that calcium silicates stimulate the formation of hydroxyapatite (HA) onto the surface of the supplying material and provides a biologic seal.9
TheraCal LC’s hydrophilic resin formula is unique. It is permeable to dentinal fluid but relatively insoluble to resist dissolution. TheraCal LC may act or resemble a scaffold for dentin formation. Dentinal fluids are readily absorbed within it resulting in the release of calcium and hydroxide ions. Immediately one of the tooth’s responses is to form apatite (HA) to the undersurface of TheraCal LC potentiating the natural sealing ability of the product. Its biocompatibility and ability to assist in the formation of apatite may play a critical and positive role in pulpal protection.7-12
TheraCal LC is dispensed via its flowable syringe directly onto moist affected dentin or exposed pulp tissue. Hand mixing, instrument placement, or triturating is not needed. It is light-cured at 20-second impulses in up to 1-mm increments. The rapid calcium release assists in stimulating the tooth’s natural healing process.
Direct-Composite Placement
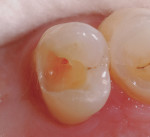
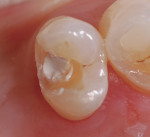
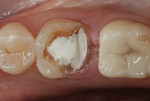
In this case, the clinician performed a direct-composite restoration for a patient presenting with an asymptomatic direct carious exposure. Light bleeding was controlled with sterile saline compression (Figure 1). TheraCal LC was placed directly over the exposure site, light-cured for 20 seconds, and then additional increments were added to seal and provide a barrier for healing (Figure 2).
Indirect Pulp Capping
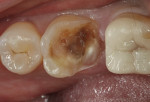
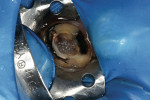
TheraCal™ LC can also be used for indirect pulp capping. Infected soft dentin under a previously placed restoration was removed, leaving affected dentin that could easily be cleaned with CAVITY CLEANSER™ (2% chlorhexidine, BISCO) (Figure 3).
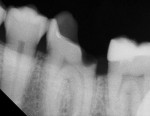
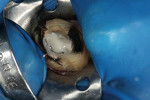
Radiographic interpretation shows the close approximation of an asymptomatic pulp (Figure 4). TheraCal LC was placed onto moist dentin in up to 1-mm increments and immediately light-cured. ALL-BOND SE® (BISCO) was subsequently placed to bond CORE-FLO™ DC (BISCO) as a base/core (Figure 5). Both TheraCal LC and CORE-FLO DC are radiopaque, allowing these materials to be easily identified on radiographs (Figure 6).
Sealing of Root Orifices and Dentin at the Furcation Floor
TheraCal LC also protects endodontically treated teeth. In this example, endodontic re-treatment was completed first. The chemically softened, disinfected furcation floor required sealing of root orifices and softened dentin at the furcation floor (Figure 7). TheraCal LC was added in 1-mm increments to provide a visually discernible orifice and furcation floor seal (Figure 8).
Conclusion
Dentists may consider TheraCal LC as a new step toward taking what research understands and creating improved materials that address the needs of healing and pulpal protecting (dentinal bridging and dentin remineralization) those of sustained alkalinity, calcium stimulation, immediate sealing to assist against bacterial invasion, and the physical strength to hold up under pressure.
References
1. Foley J, Evans D, Blackwell A. Partial caries removal and cariostatic materials in carious primary molar teeth: A randomised controlled clinical trial. Br Dent J. 2004;197(11):697-701.
2. Mertz-Fairhurst EJ, Curtis JW Jr, et al. Ultraconservative and cariostatic sealed restorations: results at year 10. J Am Dent Assoc. 1998;129(1):55-66.
3. Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009;34(5):615-625.
4. Demarco FF, Tarquinio SB, Jaeger MM, et al. Pulp response and cytotoxicity of 2 dentin bonding agents. Quintessence Int. 2001;32(3):
211-220.
5. Cehreli ZC, Turgut M, Olmez S, et al. Short term human primary pulpal response after direct pulp capping with fourth-generation dentin adhesives. J Clin Pediatr Dent. 2000;25(1):65-71.
6. Kamitakahara M, Kawashita M, Kokubo T, Nakamura T. Effect of polyacrylic acid on the apatite formation of a bioactive ceramic in a simulated body fluid: fundamental examination of the possibility of obtaining bioactive glass-ionomer cements for orthopaedic use. Biomaterials. 2001;22(23):3191-3196.
7. Hebling J, Costa C, Nogueira I, et al. Cytotoxicity of resin-based light-cured liners. Am J Dent. 2009;22(3):137-142.
8. Cannon M, Gerodias I, Vieira EM, et al. Effects of Different Pulp Capping Techniques on Hard Tissue Bridge Formation, European Academy of Pediatric Dentistry. June 2006, Amsterdam, Netherlands.
9. Mineral Trioxide Aggregate, Comprehensive Literature Review, Journal of Endodontics, March 2010.
10. Gandolfi MG, Suh B, Siboni F. Chemical-physical properties of TheraCal LC pulp capping material. Presented at: International Association of Dental Research (IADR). March 18, 2011; San Diego, CA. Abstract #2521.
11. Gandolfi MG, Siboni F, Taddei P, et al. Apatite-forming ability of TheraCal LC pulp capping material. Presented at: International Association of Dental Research (IADR). March, 2011; San Diego, CA. Abstract #2520.
12. Gandolfi MG, Siboni F, Taddei P, et al. Chemical-physical properties of TheraCal LC, a novel light-curable MTA-like material for pulp-capping, International Endodontic Journal, 2012.
About the Author
Douglas J. Brown DDS, FAGD
Director of Clinical Education
BISCO, Inc.
Schaumburg, Illinois