Optimizing Root Coverage With L-PRF
The allogeneic and xenogeneic tissues have shown excellent results in the short term.
By Robert A. Horowitz, DDS
Esthetic results are one of the objectives of coronal advancement of the gingival margin. Obscuring the margin of a tooth- or implant-supported restoration or leveling the smile line are some of the aims of periodontal plastic surgery. Often abfraction, abrasion, or carious lesions will render the exposed root surface more sensitive to thermal and chemical stimuli. In addition, restorative materials may not remain where placed in smaller lesions. These indications often drive a patient to have the area treated biologically.
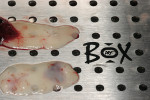
There are many products and methods for augmenting gingival tissues around natural teeth. These range from autogenous grafts (ie, subepithelial connective tissue, free gingival tissue) to allograft products (processed dermis, amnion, and pericardium). Xenogeneic and synthetic materials are used in the periodontal plastic surgical arena. Biologic activating factors and wound enhancers such as bone morphogenetic protein 2 (BMP-2), platelet-rich plasma (PRP), leucocyte- and platelet-rich plasma (L-PRF) (Figure 1), recombinant human platelet-derived growth factor (rhPDGF bb), and enamel matrix proteins are often used in the procedure.
More than 40 years ago, questions were raised on the biologic and physiologic needs for keratinized gingiva at the margin of teeth. Most periodontists prefer giving patients the ability to maintain restorations around teeth and implants surrounded by a zone of pink, attached, keratinized tissue. It has been shown that when there is < 2 mm of keratinized tissue around teeth, there is more gingival inflammation.1 Increased inflammation and plaque accumulation have been found around implants with non-keratinized marginal mucosa.2 These superficial soft tissue signs were not associated with additional loss of alveolar bone. However, there have been no definitive studies demonstrating longer retention of teeth, implants, and/or the restorations on them if the marginal gingival is mucosa or keratinized in histologic composition.
Harvesting of autogenous tissue from the maxillary arch requires involvement of a second surgical donor site; removing a free gingival graft from the palate leaves a large, bleeding wound site.3,4 Significant shrinkage of the grafts has also been noted. Although the connective tissue in the tuberosity area is thick, the quantity of material available for harvest is often limited.5 In an attempt to decrease these and other complications, grafts have been harvested from inside the palatal soft-tissue flap. In this way, connective tissue can be harvested in a subepithelial manner.6 This technique gives the surgeon the ability to obtain a significant quantity of soft tissue and leave the patient with minimal discomfort and bleeding.
In an attempt to increase patient acceptance of gingival augmentation procedures, allogenic tissues have been used.7 When the surgeon takes a product “off the shelf,” there is an unlimited supply of material to treat multiple recipient sites. On the other hand, these products have minimal to no inductive activity. For this reason, blood-concentrate products have been recently used alone8 or added to acellular dermal grafts in an attempt to improve healing.9 In some of the studies, there has been an improvement in patient perception of the initial healing sequelae. The cases shown in this evaluation will demonstrate some of the uses of leucocyte-rich platelet-rich fibrin (L-PRF) with the addition of a resorbable barrier for root coverage. The blood-concentrate technique used in these cases is simpler to fabricate than some others on the market. Studies have also shown that there is a longer release of growth factors from the fibrin clot produced by the L-PRF method.10,11 The combination of materials may yield a sustained release for a longer time period in the surgical site. This has the potential of maximizing healing and root coverage in a deficient area.
Case Reports
Patient 1
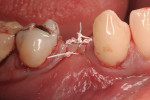
This patient presented with a narrow alveolar ridge in an edentulous site (Figure 2). The adjacent teeth each had a 3-mm-deep x 5-mm-wide facial recession with sensitivity to extreme temperature. Cone-beam computed tomography demonstrated sufficient alveolar bone in which to place a tapered endosseous implant, fully embedded in bone. In an attempt to augment the sites at the time of implant placement, a combination soft- and hard-tissue enhancement was performed at the same visit. Two 9-mL tubes of blood were drawn from the patient’s arm. No anticoagulants or other chemicals were added to the material and it was centrifuged at 2,700 rpm for 12 minutes (PRF Process, Intra-Lock International, Inc., www.intra-lock.com), then the concentrated L-PRF was removed from the tube (Figure 3). This mass was placed on top of the resorbable collagen barrier (DynaMatrix®, Keystone Dental, Inc., www.keystonedental.com) to allow the liquid-containing growth factors to be absorbed by the barrier.
After placement of an endosseous implant (PrimaConnex®, Keystone Dental, Inc.) in an ideal location with no threads exposed through bone on the facial surface, an allograft putty (Dynablast™, Keystone Dental, Inc.) was used to rebuild the site to ideal contour on the facial of the alveolar ridge. The graft, recession sites, and coronal portion of the implant were then covered with the “activated” barrier (DynaMatrix, Keystone Dental, Inc.). This was covered facially by one layer of L-PRF over the tooth roots. The other layer of the fibrin was placed from the buccal–gingival extent of the barrier under the lingual flap (Figure 4 and Figure 5). Primary closure was obtained after releasing the facial flap with PTFE sutures (Cytoplast® 5-0, Osteogenics Biomedical, www.osteogenics.com) (Figure 6). The area healed uneventfully, with no postoperative hematoma and no need for narcotic analgesics.
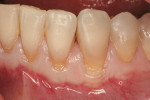
Six months after the implant was placed, a minimally invasive uncovering was performed. The site was restored 2 months later with a custom abutment and a cemented ceramometal restoration. The gingival height, contours, and papillae have been maintained through the 2-year follow-up time (Figure 7).
Patient 2
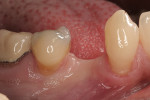
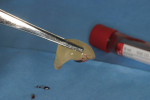
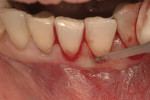
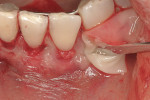
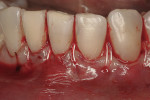
This 50-year-old physician presented with multiple sites of root sensitivity associated with gingival recession (Figure 8). She had very thin, scalloped biotype tissue, minimal keratinized tissue, and the demand for no palatal tissue to be harvested. After root preparation with aggressive scaling and tetracycline application, sulcular incisions were made with a microsurgical blade (Figure 9). This freed up the facial flaps up to the crest of the alveolar ridge. The exposed root surfaces were treated with tetracycline, thoroughly irrigated, and coated with enamel matrix proteins (Straumann® Emdogain, Straumann USA LLC, www.straumann.us). The fibrin material was placed in the PRF Box (Intra-Lock International, Inc.), and the metal cover was placed on it, expressing the liquid exudate into the bottom of the box. A 1-cm-high x 30-cm-wide piece of pericardial tissue (Pericardium, RTI Biologics, Inc., www.rtix.com) was wetted with the liquid from the PRF box. Once “activated” by the liquid for 15 minutes, the pericardial tissue was inserted through the split-thickness facial flap in a tunnel approach (Figure 10).12 The material was stabilized mesially, distally, and interproximally with resorbable gut sutures. The facial flap was coronally sutured with 5-0 PTFE sutures to bring the gingival margin to the level of the cementoenamel junction (CEJ) (Figure 11).
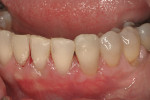
The site was debrided at 1-, 2-, and 4-weeks postoperatively. At the 2-week visit, the sutures were removed. After the surgical procedure, the patient reported minimal swelling for 2 days and no need for analgesic medications. The site has healed well to the 6-month follow-up (Figure 12). All symptoms of thermal reaction have disappeared, the gingival margins are at the CEJ of the treated teeth.
Patient 3
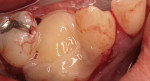
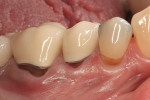
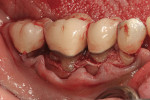
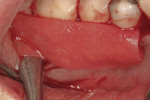
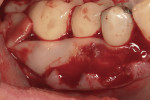
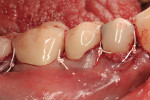
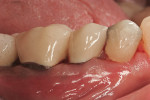
Severe facial abfraction lesions were present on the facial surfaces of teeth Nos. 28 to 30, associated with temperature sensitivity and food impaction (Figure 13). A full split-thickness facial flap was elevated with no vertical releasing incisions. A high-speed rotary instrument13 was used to reduce the buccal contours and eliminate the notching (Figure 14) in the affected areas. Removal of the smear layer and surface demineralization to expose collagen fibers was accomplished with the use of a tetracycline slurry paste. After thorough removal of this treatment, the root surfaces were treated with Emdogain. Alveolar defects were grafted with a mixture of anorganic bovine bone mineral (Bio-Oss®, Osteohealth® Company, www.osteohealth.com). The area was covered with a 10-mm x 30-mm piece of bilayer porcine collagen (Mucograft®, Osteohealth Company) soaked in the liquid expressed from PRF clots placed in the PRF box (Figure 15 and Figure 16). The collagen was then covered with a PRF membrane, and the vestibular flap was repositioned to the crown margins and CEJ (Figure 17). The sutures were removed at 2 weeks. The image shown is 8-week healing with an excellent soft-tissue response (Figure 18).
Discussion
Patient demands for esthetic, functional results from periodontal procedures continue to raise the standards to which surgeons must perform. Numerous techniques and materials are available to the dentist, who must choose which meet the specific demands of the site to be treated. Although autogenous grafting of subepithelial connective tissue has been shown in multiple studies to yield predictable, esthetic results, not all patients have enough volume of this tissue or are willing to allow the palate to be harvested.
PRP has been used in dental procedures for more than 10 years.14 Although advocated as an enhancer for bone and soft-tissue grafts, the results have been varied and, in general, not statistically significant in terms of increased bone or gingival formation when used. The PRP liquid may be difficult to use in certain procedures and the growth factors are released primarily into solution over the first day.15 Other studies have shown that L-PRF has the ability to progressively release the growth factors incorporated in its fibrin matrix remodeling.16 A sustained, 7- to 10-day release of the incorporated growth factors explains why better healing results have been obtained with L-PRF than other blood-concentrate products.16
L-PRF was first studied as a wound-healing enhancer in oral and maxillofacial surgery 10 years ago.17 In solution, fibrinogen is the alpha granules of platelets and in plasma, it is an important part of the aggregation of platelets in the hemostasis cascade. It is produced in a simpler manner than PRP, requiring less time and fewer materials. After drawing 10-mL tubes of blood with no additives,18 it is centrifuged for 10 minutes at 3,000 rpm. The fibrin clot is formed in the central portion of the tubes after the thrombin present in the blood mixes with the fibrinogen released by the activated platelets. This clot contains serum and platelets in a homogenous 3-dimensional (3-D) matrix.19 Because of the slow polymerization, the resulting elastic architecture releases cytokines and growth factors trapped in the matrix over a long time. All of these aspects result in the ability to call PRF a healing biomaterial, not just a fibrin adhesive.20 The combination of the leukocytes trapped in the 3-D matrix of PRF and the cytokines released gradually during the healing process both contribute to the acceleration of the healing process.21
Mucograft is a bilayer, porcine collagen barrier. The thick 3-dimensionality facilitates adsorption of the initial blood clot formed in a surgical site and/or liquid growth factors or enhancers that it may be soaked in. As the initial clot forms and is stabilized in this area, wound healing can progress in an undisturbed manner and at a rapid rate. The more superficial portion is denser from the compact fibers, delaying epithelial or bacterial migration into the deeper layers. As a stiffer and less quickly biodegradable portion of the barrier, the structure of the entire complex is maintained in an ideal shape for a longer period of time.22 This can help explain the beneficial early results in root coverage studies.23,24 Hydration of this material with a liquid “growth factor” should enable a timed release of the active factors. Clinical results from studies already published show similar results to those in which autogenous tissues were used.24 In general, the patients preferred the simpler, one surgical site approach when the surgeon used nonpalatal tissue as the donor.
Pericardial tissue is harvested from cadavers and processed by differing methods depending on the company. The specific pericardium used in the cases shown here is processed by a proprietary process (Tutoplast® Tissue Sterilization Process, RTI Biologics, Inc.). Before procurement and processing, the potential donors are screened for potentially infectious agents.25 The tissues are treated to have lipids and bacteria removed, are deproteinated and any possible prions are eliminated in an acetone bath.24 The resulting barrier is nonantigenic, very strong, and able to be hydrated in any of a number of liquids allowing insertion in the soft-tissue deficient site in a tunneled manner. This enables the surgeon to augment the involved site with a minimum of surgical trauma to the patient.
The patients in this case series had blood drawn and processed for PRF. The liquid expressed from the PRF was used to hydrate the graft tissue. This exudate has been shown to have high levels of various growth factors, which are beneficial in wound healing.10,18,26 The “growth factors” of L-PRF were then able to be added to other biomaterials. The graft material placed in the defects has been used in sinus augmentation and shown to result in vital bone formation.27 The barrier was more effective than other resorbable barriers in both vital bone formation and in the lack of inflammation.28 The addition of these autogenous growth factors has been successful in these cases mixed with and enhancing the biological effects of a wide range of products.
Allograft tissues have been studied for root coverage and esthetic improvement in edentulous and implant-related sites for a number of years.29,30 Materials such as amnion and chorion contain growth factors and binding proteins, which enhance wound healing in ophthalmic and periodontal sites.31,32 The addition of growth-enhancing materials and endogenous growth factors may enhance the results with other graft and barrier combinations, which are nonvital9 and have no binding or other growth-factor characteristics. The percentage of root covered and esthetic results with the new combinations demonstrated in this case series is impressive, approaching that of multiple studies with enamel matrix derivative33 and/or connective tissue grafts.34 The sites treated in this series demonstrate other methods of addressing the issues of exposed roots and deficient keratinized tissue enhanced with autologous growth factors that can be released in the surgical site over an extended time period.11 Although these methods may be less invasive than other approaches, the clinical results obtained are still ideal.
Conclusion
Many patients prefer a method of gingival augmentation that does not require harvesting their own tissue from the palatal area. The allogenic and xenogeneic tissues used here have shown excellent results in the short term. Continuing research along these lines, supported with histologic validation of true periodontal regeneration,35,36 will enable periodontal surgeons to continue to deliver optimal care. As more of these answers become available, the periodontal surgeon will have an expanded armamentarium of techniques and materials to predictably, biologically answer more surgical challenges.
Acknowledgment
The author would like to thank Dr. Peter Nordland for his insights and modification of “current” periodontal plastic surgery procedures, and Ms. Kary Ann Clarke and Gayla Miller for their preparation of the PRF.
References
1. Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623-627.
2. Chung D, Oh T, Shotwell J, et al. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol. 2006;77(8):1410-1420.
3. Silva C, Ribeiro Edel P, Sallum AW, et al. Free gingival grafts: graft shrinkage and donor-site healing in smokers and non-smokers. J Periodontol. 2010;81(5):692-701.
4. Miller PD Jr. Root coverage using a free soft tissue autograft following citric acid application. Part 1: technique. Int J Periodontics Restorative Dent. 1982;2(1):65-70.
5. Jung UW, Um YJ. Choi S. Histologic observation of soft tissue acquired from maxillary tuberosity area for root coverage. J Periodontol. 2008;79(5):934-940.
6. Griffin TJ, Cheung WS, Zavras AI, et al. Postoperative complications following gingival augmentation procedures. J Periodontol. 2006;77(12):2070-2079.
7. Wang HL, Bunyaratavej P, Labadie M, et al. Comparison of 2 clinical techniques for treatment of gingival recession. J Periodontol. 2001; 72(10):1301-1311.
8. Aroca S, Keglevich T, Barbieri B, et al. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol. 2009;80(2):244-252.
9. Shepherd N, Greenwell H, Hill M, et al. Root coverage using acellular dermal matrix and comparing a coronally positioned tunnel with and without platelet-rich plasma: a pilot study in humans. J Periodontol. 2009:80(3):397-404.
10. Dohan Ehrenfest DM, Peppo GM, Doglioli P, et al. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009:(27);63-69.
11. He L, Lin Y, Hu X, et al. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro, Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):707-713.
12. Nordland WP, Sandhu HS, Perio C. Microsurgical technique for augmentation of the interdental papilla: three case reports. Int J Perio Restorative Dent. 2008;6(28):543-549.
13. Evian CI, Horowitz RA, Karateew ED, et al. Evaluation of a surgical debridement bur for use in periodontal surgery. Compend Contin Educ Dent. 1998;19(11):1124-1132.
14. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
15. Lucarelli E, Beretta R, Dozza B, et al. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13-23.
16. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):51-55.
17. Choukroun J, Adda F, Schoeffler C, et al. Uneopportunité en paro-implantologie: le PRF. Implantodontie 2000;42:55-62.
18. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):37-44.
19. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):45-50.
20. Gaultier F, Navarro G, Donsimoni J-M, et al. Platelet con- centrates. Part 3: Clinical applications. Implantodontie 2004;13:3-11.
21. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):56-60.
22. McGuire MK, Scheyer ET. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J Periodontol. 2010;81(8):1108-1117.
23. Sanz M, Lorenzo R, Aranda JJ, et al. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J Clin Perio. 2009;36(6):523-531.
24. Herford AS, Akin L, Cicciu M, et al. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J Oral Maxillofac Surg. 2010;68(7):1463-1470.
25. Brown P. Rohwer RG. Gajdusek DC. Newer data on the inactivation of scrapie virus or Creutzfeldt-Jakob disease virus in brain tissue. J Infect Dis. 1986;153(6):1145-1148.
26. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:51-55.
27. Kim DM, Nevins ML, Camelo M, et al. The efficacy of demineralized bone matrix and cancellous bone chips for maxillary sinus augmentation. Int J Periodontics Restorative Dent. 2009;29(4):415-423.
28. Sommerlad S, Mackenzie D, Johansson C, et al. Guided bone augmentation around a titanium bone-anchored hearing aid implant in canine calvarium: an initial comparison of two barrier membranes. Clin Implant Dent Relat Res. 2007;9(1):22-33.
29. Allen EP. AlloDerm: an effective alternative to palatal donor tissue for treatment of gingival recession. Dentistry Today. 2006;25(1):48-52.
30. Rahmani ME, Lades MA. Comparative clinical evaluation of acellular dermal matrix allograft and connective tissue graft for the treatment of gingival recession. J Contemp Dent Pract. 2006;7(2):63-70.
31. Velez I, Parker WB, Siegel MA, et al. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 2010;81(12):1797-1804.
32. Horowitz RA. Gingival augmentation. Inside Dentistry 2009;60-66.
33. Pilloni A. Paolantonio M. Camargo PM. Root coverage with a coronally positioned flap used in combination with enamel matrix derivative: 18-month clinical evaluation. J Periodontol. 2006;77(12):2031-2039.
34. McGuire MK, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: comparison of clinical parameters. J Periodontol. 2003;74(8):1110-1125.
35. McGuire MK, Cochran DL. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: histological evaluation. J Periodontol. 2003;74(8):1126-1135.
36. Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J Clin Periodontol. 1997;24(9 Pt 2):693-696.
About the Author
Robert A. Horowitz, DDS
Clinical Assistant Professor
Departments of Periodontology and Implant Dentistry
New York University College of Dentistry
New York, New York
Private Practice
Scarsdale, New York