Crown Repair Using Glass Ionomer
John C. Comisi, DDS
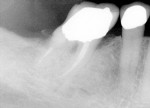
An 82-year-old man presented for routine dental cleaning and examination. Tooth No. 30 had a composite restoration that was placed several years prior in an attempt to repair an existing crown on this endodontically treated tooth (Figure 1).
Another dentist that the patient had consulted with previously recommended that the tooth be removed. The tooth was still functional, and the patient really wanted to find a way to “keep it working.” Removal was out of the question for the patient. Was there a way to do as the patient asked? Was there an acceptable way to do it?
It certainly was a challenge. Was there a method to make this work? A solution needed to be found. Composite was used on this tooth in the past and evidently worked for a period of time. However, the potential for further marginal leakage, which unfortunately would reoccur with this approach, was just not acceptable. Amalgam? Perhaps; however, having not placed an amalgam intraorally in the past 6 years, there was none available in the author’s office.
Over the past few years, various glass ionomer and resin-modified glass-ionomer products have taken on an expanded role in the office, at times, as a restorative material in combination with composite materials. The results have been fabulous.
Why glass ionomers? They are a bioactive material with an affinity to tooth structure. Typically, when placing a glass ionomer a weak acid or conditioner is used to aid in releasing calcium and phosphate ions from the tooth structure. These calcium and phosphate ions combine into the surface layer of the glass ionomer and form an intermediate layer called the interdiffusion zone.1 This bond layer can be very strong and significantly reduce the microleakage that would occur at the margins of the restoration.2
Glass ionomers have other properties that make them a very versatile restorative material3-10:
- Very good fluoride and ion release helps remineralize tooth structure in the remineralization–demineralization process that naturally occurs in the oral cavity.
- They bond to enamel, dentin, and metals.
- They produce good marginal integrity.
- They shrink only one ninth the amount of composite material.
- They are fluoride-rechargeable.
- There are no free monomers in the material.
- The cavity preparation can be bulk-filled, making the materials easy to place.
- They exhibit excellent biocompatibility.
Thus, it was determined that in this situation it would be very worthwhile and beneficial to use a glass ionomer to restore tooth No. 30.
Clinical Procedure
Rotary instrumentation was used to remove the old composite restoration on the distal portion of tooth No. 30, as well as any structure that was soft and decaying. After instrumenting into solid structure, the preparation was etched with a cavity conditioner, a weak acid (usually polyacrylic acid can be used), for 10 seconds. The area then was washed and dried, but not excessively because the area should not be desiccated.
Meanwhile, the glass ionomer, in this case Fuji IX GP Fast (GC America, Inc, Alsip, IL) in capsule form, was mixed for 10 seconds in a triturator and then placed into a capsule applicator gun. Then the material was “injected” into the cavity preparation. A flat-sided plastic instrument and ball burnisher were used to adapt the material into the preparation site. The instrument ends were moistened lightly with water on a gauze sponge by the assistant. This aids with adaptation and manipulation of the glass ionomer. It is important not to over-moisten the instrument. Fuji IX GP Fast was chosen because it can be packed into the preparation, and because it can fill the preparation densely.
Fast-setting glass ionomer will generally set in approximately 3 minutes. However, in many instances, 3 minutes can feel like a lifetime. Clinicians can accelerate the set of these types of glass ionomer by using heat. Dr. Geoff Knight, who has done incredible work with glass ionomers in Australia, has presented in his lectures that by using a bleaching light one can help accelerate the setting of glass ionomers, so that they can set in less than 1 minute. The author used the bleaching tip attachment for the Radii Plus (Southern Dental Industries, Inc, Bensenville, IL) to accelerate the cure to approximately 50 seconds (Figure 2 and Figure 3). The attachment is the same size as the curing tip (~ 8 mm) and is able to be rotated 360º, so reaching the hard-to-reach areas becomes easier.
Then an unfilled resin (both GC America, Inc, and Southern Dental Industries, Inc, have a coat material for this purpose) was applied and light-cured with the Radii Plus curing tip for 10 seconds. The excess material was trimmed away, making the restoration flush with the tooth structure. After polishing, an additional layer of unfilled resin was placed and light-cured for 10 seconds.
The final restoration (Figure 4) showed excellent adaptation and filled the preparation. The bioactive nature of the glass ionomer should help reduce the potential for secondary decay. The use of glass ionomer will make it easy to monitor the effectiveness of the seal created by the restoration to the tooth both clinically through observation and radiographically, because the material is nicely radio-opaque. And, of course, the patient was very pleased with the result and the fact that a way was found to help extend the lifespan of this tooth.
Disclosure
Dr. Comisi is a consultant for SDI, North America, Inc. He has received no monetary compensation from SDI or GC America for the information provided in this article.
References
1. Van Meerbeek B, De Munck J, Yoshida Y, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215-235.
2. Frencken JE, Van’t Hof MA, Van Amerongen WE, Holmgren CJ. Effectiveness of single-surface ART restorations in the permanent dentition: A meta-analysis. J Dent Res. 2004;83: 120-123.
3. Swartz ML, Phillips RW, Clark HE. Long-term F release from glass ionomer cements. J Dent Res. 1984;63: 158-160.
4. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 16(2):129-138.
5. Cenci MS, Pereira-Cenci T, Donassollo TA, et al. Influence of thermal stress on marginal integrity of restorative materials. J Appl Oral Sci [online]. 2008;16(2): 106-110.
6. Hewlett ER, Mount GJ. Glass ionomers in contemporary restorative dentistry—a clinical update. J Calif Dent Assoc. 2003;31(6): 483-492.
7. Kasuyama S, Ishikawa T, Fujii B. Glass ionomer dental cement: the materials and their clinical use. St. Louis: Ishiyaku, EuroAmerica Inc; 1993:53-59.
8. Wilson AD, McLean JW. Glass ionomer cements. Chicago: Quintessence; 1988:126-128.
9. Tyas MJ. Reaction and discussion: clinical performance of glass ionomer cements. Symposium on esthetic restoration materials. Chicago: American Dental Association; 1991.
10. Davidson CL. Advances in glass-ionomer cements. J Appl Oral Sci [online]. 2006;14:3-9.
About the Author
John C. Comisi, DDS
Private Practice
Ithaca, New York