Resin Cements: A Closer Look at Newly Introduced Cements
Janet L. Harrison, DDS; Waldemar G. de Rijk, PhD, DDS; and James F. Simon, DDS, MEd
Resin cements continue to evolve with refinements engineered by the manufacturers. One of the big changes in recent years has been the development of all-in-one resin cements, which are said to eliminate the need for a dentin bonding system when placing indirect restorations. These cements are variously la-beled as all-in-one cements, universal cements, self-adhesive cements, or adhesive cements. The classification by the manufacturers is not always clear, because traditional resin cements are now called adhesive cements as well.
Nevertheless, it appears that there are now two groups of resin cements: a group that requires a dentin bonding system before use and a group of cements for which the use of a bonding agent is optional. The authors would like to refer to the cements in the second group as self-adhesive resin cements. The bonding mechanism of these resins is based on an acid-base link to the calcium in hydroxyapatite in dentin and enamel while maintaining the physical properties of resin cement—an approach that makes the bonding to dentin similar to a resin-modified glass-ionomer cement without the need of the glass-ionomer component.1 A list of current self-adhesive resin cements is given in Table 1.
PHYSICAL PROPERTIES OF RESIN CEMENTS
There exists an international standard, ISO 4049, that sets forth a group of minimum standards for the physical properties of resin cements,2 including the self-adhesive cements. These standards are identical to the ANSI/ADA specification No. 27.3 The ISO standard addresses primarily resin-based composites, but includes specific requirements for resin-based luting agents. Even though the ISO standard is a voluntary one, many manufacturers of resin cements are in compliance with the ISO standard and indicate that on the package or the enclosed literature. The ISO standard requires a minimum value for the transverse flexural strength (50 MPa), radiopacity (equivalent to aluminum), and a limit on solubility in water. At this time there is no requirement on adhesive properties; however, most manufacturers report adhesive strength as measured by the shear bond strength test.4 In selecting a resin cement, compliance with the ISO standard is a good starting point.
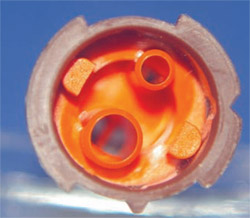
COMPOSITION OF RESIN CEMENTS AND SELF-ADHESIVE RESIN CEMENTS
Resins in this case are defined as blends of polymerizable monomers of methacrylates, dimethacrylates, and polymethacrylates (which have recently been included into the blend as well). For the self-adhesive resin cements, additions to the resin blend consist of highly acidic resin monomers, monomethacrylates, multifunctional methacrylates, and at least one of the new cements includes 4-methacryl-oxyethyl trimellitic acid (4-MET). The inclusion of the acidic monomers creates a self-etching resin, increasing the adhesive properties. This concept of self-etching finds its origin in the development of the self-etching adhesives. Further additives are included to increase the bond strength to metals (eg, 4-MET) and to resins that bond to ceramic interfaces.
The first highly acidic monomer was an acrylic chain on which phosphoric acid groups were grafted. Since then, other acidic monomers have been introduced. The goal is to include the dentin bonding agent and a ceramic bonding system or metal bonding system into the resin cement and have the entire assembly polymerized at once.
Several manufacturers have included a fluoride component as well.5,6 This practice is of dubious value, as the acrylate resins are very hydrophobic. The mechanisms of fluoride release and of maintaining low water absorption are mutually exclusive. Cement that meets the ISO standard on water solubility cannot release a clinically effective amount of fluoride. One manufacturer carefully states that its product "contains" fluoride and claims a release of 7 ppm to 9 ppm independent of the exposed surface area.5 In the authors’ opinion, whether a cement contains fluoride or not should not be a factor in the product selection of resin-based cements.
For two of the products in Table 1 (EMBRACE™ Wetbond™, Pulpdent Corporation, Watertown, MA; and MonoCem™ Self-Adhesive Resin Cement, Shofu Dental Corporation, Menlo Park, CA), it is recommended that the cement be applied to moist dentin, not dry dentin.5,6 It is claimed that the uncured resin is hydrophilic, but that the cured resin is hydrophobic.7 The authors hope to investigate this claim and report the findings in the near future.
The viscosity of the resin cements have been significantly reduced over the last few years. The reason for the change is primarily the introduction of new delivery systems (see next section). To achieve these low viscosities, the particle size of the inorganic fillers has been greatly reduced. Most cements now have an average particle size well below 1 µm. Submicron or nanofiller particles are becoming a significant component of resin cements and composites. The filler content of the cement is kept at or below 50% by volume. The second step in reducing the viscosity was obtained by using very low molecular weight monomers as diluents (2-hydroxyethylmethacrylate [HEMA], tetraethyleneglycol dimethacrylate [TEGDMA]). The result of using these diluents is that the polymerization shrinkage of the cement increases to about 4% by volume (1.2% linearly), which is of little consequence for cement.
DELIVERY SYSTEMS OF RESIN CEMENTS
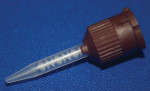
During the past few years, the delivery system of resin cements has drastically changed—from kits containing multiple single syringes that require mixing on a mixing pad to dual syringes with a static mixing tip. Other delivery systems include premeasured capsules and double-barreled syringes for equal dispensing that still require mixing by hand. One cement (RelyX™ Unicem Self-Adhesive Universal Resin Cement, 3M ESPE, St. Paul, MN), which was previously only available in premeasured capsules and required trituration, has now become available in a dual syringe without a mixing tip (Figure 1). This system requires hand mixing, but eliminates the awkward capsule dispenser.
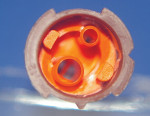
Static mixing tips mix the two resin components by swirling the resins in alternating directions. When traversing the tip, the direction of rotation changes at least three times. For most cements the direction changes five times; for some cements this number can be as high as nine times. The development of the static mixer was in part made possible by computer modeling of fluid flow in three dimensions. In Figure 2, a computer model of a static mixer is shown.
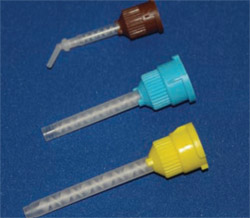
It should be kept in mind that not all static mixing tips are created equal. Mixing tips come with a different number of mixing stages (Figure 3; Figure 4; Figure 5). The mixing tips in Figure 5 are interchangeable, thus making it possible to put the wrong tip on a syringe. The tips are optimized for the product for which they are designed and using the wrong tip can lead to a very poorly mixed cement (too few mixing stages) or a very fast-setting cement that delivers too slowly (too many mixing stages). The color coding of mixing tips (brown in the case of Figure 3; Figure 4; Figure 5) refers to the size of the cartridge for which the tip was designed, not to the number of mixing stages.
To complicate matters even more, some mixing tips are designed for a mixing ratio of 1:1 (Figure 6) and others are designed for a mixing ratio of 2:1 (Figure 7). Knowing this, it can be seen that relying on the color of the mixing tip is not a good idea. It is recommended that only the mixing tips that came with the cement be used for that product.
For one product, the manufacturer decreased the viscosity of a core material in increments. The material maintained the same name and application, but as can be seen in Figure 8, mixing tip A was introduced in 2001. In 2003 the company released the material with mixing tip B, and in 2006 the material was introduced with mixing tip C. Tips A and B were for large cartridges that required a dispensing gun and tip C is for a small cartridge without a dispensing gun. This illustrates how the viscosities of resin-based materials are being changed continually to accommodate the new delivery systems. For a material that is intended for intraoral delivery, the development of tip C is obviously a major improvement in the delivery system.
STRENGTH OF CEMENTS
Do these new cements work? When the restoration is ready to deliver, two things are important for the success of the restoration: the design of the preparation and the cement that is to be used. Resistance form is defined as the features of a tooth preparation that enhance the stability of a restoration and resist dislodgement along an axis other than the path of placement.8 Resistance form can overcome many of the shortcomings of the cement.
An increase in the taper and a decrease in the height of the preparation affect the retention that the preparation can add to the long-term success of the restoration. Retention depends upon the shape of the preparation; and, with good retentive features, any traditional nonadhesive luting material will provide long-term success. It was determined that at a taper of greater than 26°, the retentive value of the preparation was zero.9 In another study, increasing the taper from 6° to 24° decreased the retention with the four luting agents tested: zinc phosphate by 42%, glass ionomer by 38%, PANAVIA (Kuraray America, Inc, New York, NY) by 20%, and C&B?Metabond® (Parkell, Inc, Edgewood, NY) by 10%, and they recommended that any preparation with a compromised taper of over 12° should be bonded to improve the chances of crown retention and clinical success.10
Two studies looked at the retentive strength of zirconium-oxide ceramic crowns cemented with different cements. One study found similar retentive values for the 12 cements tested, but their standardized preparation was 5°. A great deal of their retention was probably coming from the preparation itself and might account for the similarity of results.11 The other study used a preparation of 20° and also had similar results for the three cements tested (a composite resin PANAVIA F 2.0 at 5.1 MPa; a resin-modified glass-ionomer, RelyX™ Luting by 3M ESPE, at 6.1 MPa; and a self-adhesive modified composite, RelyX Unicem, at 5 MPa).12
BOND STRENGTH STUDIES
Many of the researchers report bond strength studies that manufacturers use in their advertisements to support the use of their cement when comparing it to another company’s cement. The data is presented as shear bond tests or as microtensile bond tests (these numbers are usually reported as larger than shear bond tests because of the nature of the tests) to human or bovine dentin, enamel, and different materials. All of this can be confusing—and sometimes misleading. Usually, these published numbers are much lower than numbers normally seen when testing dentin bonding systems and vary greatly depending upon the surface tested (enamel or dentin) and the type of test that is being conducted. For example, the published shear bond information (depending upon which company brochure one is reading or which study one finds) of RelyX Unicem varies from 4 MPa to 23 MPa on dentin,13 the data for Maxcem™ (Kerr Corporation, Orange, CA) varies from 0 MPa to 15 MPa,14 and the data for Multilink® Automix (Ivoclar Vivadent, Amherst, NY) varies from 10 MPa to 35 MPa.15 These numbers are much less than are normally seen for etched enamel (20 MPa to 25 MPa), which is the standard that dentists try to attain.
It is important to follow the manufacturer’s direction for use. Ivoclar Vivadent does not recommend that its new cement, MultiLink® Sprint, be used for non-retentive preparations or with any dentin bonding system, while RelyX Unicem is recommended for all types of restorations. Ivoclar is also now recommending that, when using its resin cement MultiLink, the A&B primer be placed on the enamel for 30 seconds and scrubbed into the dentin for 15 seconds before applying the cement.15
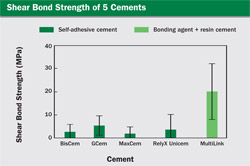
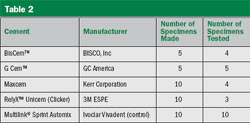
To compare several of the new self-adhesive cements to a cement that requires a bonding agent, the authors conducted the hollow-notch shear bond strength test.4 The materials tested are listed in Table 2. The UniCem cement was provided in an incrementally dispensing dual cartridge (Figure 1) and the MultiLink Automix was delivered from a dual syringe with a static mixing tip. All products were provided by their respective manufacturers. After making the specimens, they were stored in dionized water for 24 hours at 37° C, after which time the specimens were tested. Several specimens debonded during the storage time.
The test was done on a universal testing machine (model 1157, Instron, Norwood, MA) with a cross-head speed of 1 mm/min. The results are shown in Figure 9. Statistical analysis revealed that there were no differences between any of the self-adhesive cements. The difference between the self-adhesive resin cements and the control group (MultiLink with the provided bonding agent) was statistically significant at P = .05. As indicated before, the absolute values obtained in this test do not necessarily agree with the results of other laboratories; however, the variations seen between materials within a given test are reliable. From this test it is seen that as a group, the self-adhesive cements achieve a shear bond strength roughly one half of the shear bond strength obtained when a dentin bonding agent is used in conjunction with a regular resin cement. It should be pointed out that because of the low viscosity of the cements, the shear bond specimens are very difficult to produce, as very little pressure can be applied to the cement during the packing phase. The large standard deviations, as shown by the error bars in Figure 9, suggest that there are inconsistencies in the materials. It is not uncommon for self-adhesive systems, be they dentin bonding agents or self-adhesive resin cements, to show a coefficient of variation in the order of 50% or greater.
CONCLUSION
The choice of the correct cement is becoming more complicated as each manufacturer creates a new cement that is sup-posed to be better, easier, and more reliable than what has been used in the past. The lower viscosities of the cements with the static mixing tips provide thoroughly mixed cements with few voids. With many different static mixing products in a dental office, the risk exists that inadvertently mixing tips will be interchanged with unpredictable results.
It does appear that for a good bond to dentin, the use of a bonding agent is recommended when a resin cement is used. When the preparation design assists in the retention of the restoration, almost any of the new cements will work adequately. If maintenance of a good bond is critical for the success of the restoration, such as a bonded veneer, then the use of a separate bonding agent should be considered based on long-term clinical experience with bonding agents.
DISCLOSURE
The authors received material support from 3M ESPE; BISCO, Inc; GC America; Kerr Corporation; and Ivoclar Vivadent.
References
1. Simon, JF, de Rijk WG. Dental cements. Inside Dentistry. 2006;2(2):42-47.2. International Organization for Standardization Web site. Dentistry—Polymer-based filling, restorative and luting materials. ISO Standard 4049. 2000. Available at: www.iso.org/iso/en/CatalogueDetailPage.CatalogueDetail?CSNUMBER=23041. Accessed July 18, 2007.
3. Direct Filling Resins. American Dental Association Web site. ADA Specification No 27. 1993. Available at: https://www.webstore.ansi.org/ansidocstore/product.asp?sku=ADA+Specification+No%2E+27%2D1993. Accessed July 18, 2007.
4. Notched Shear Bond Test Method. CRA Newsletter Web site. Available at: www.cranews.com/additional_study/2002/02-06/UPIPROTO.htm. Accessed: July 18, 2007.
5. MonoCem™ Self-Adhesive Resin Cement. Shofu Dental Corporation Web site. Available at: www.shofu.com/cements(new).html. Accessed July 18, 2007.
6. EMBRACE™ Pulpdent Corporation. Available at: www.pulpdent.com. Accessed July 18, 2007.
7. NX3 Lit No.33675. Kerr Sybron Dental Specialties. Available at: www.kerrdental.com/index/cms-filesystem-action?file=KerrDental-PDF/nx3%20sheet%202_13_07.pdf. Accessed July 18, 2007.
8. The glossary of prosthodontic terms. J Prosthet Dent. 2005;94(1):10-92.
9. Cameron SM, Morris WJ, Keesee SM, et al. The effect of preparation taper on the retention of cemented cast crowns under lateral fatigue. J Prosthet Dent. 2006;95(6):456-461.
10. Zidan O, Ferguson GC. The retention of complete crowns prepared with three different tapers and luted with four different cements. J Prosthet Dent. 2003;89(6):565-571.
11. Ernst CP, Cohnen U, Stender E, et al. In vitro retentive strength of zirconium oxide ceramic crowns using different luting agents. J Prosthet Dent. 2005;93(6):551-558.
12. Palacios R, Johnson GH, Phillips KM, et al. Retention of zirconium oxide crowns with three types of cement. J Prosthet Dent. 2006;96(2): 104-114.
13. Data on file. 3M ESPE, St. Paul, MN.
14. Data on file. Kerr Corporation, Orange, CA.
15. Data on file. Ivoclar Vivadent, Amherst, NY.
 |  | |
| Figure 1 New incremental dual dispenser for RelyX Unicem. | Figure 2 Numerical model for flow calculations in static mixers (Image courtesy of COMSOL Inc, Burlington, MA). Animation available at: www.comsol.com/showroom/gallery/245.php. | |
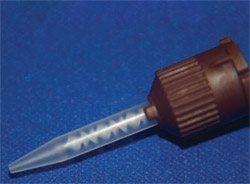 |  | |
| Figure 3 Static mixing tip with five mixing stages. | Figure 4 Static mixing tip with eight mixing stages. | |
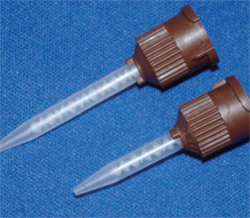 |  | |
| Figure 5 Two mixing tips with different numbers of stages that can be inadvertently interchanged, with undesirable results. | Figure 6 Inside of mixing tip for 1:1 dispensing. | |
 |  | |
| Figure 7 Inside of mixing tip for 2:1 dispensing. | Figure 8 Mixing tips for three subsequent productions of a core material (Tip A, yellow; Tip B, blue; Tip C, brown) illustrating the drastic changes in viscosity during the development of the material. | |
 | ||
| Figure 9 Bar graph of the results of the shear bond strength test. | ||
 |  | |
| About the Authors | ||
 Janet L. Harrison, DDS Janet L. Harrison, DDS Associate Professor Department of Restorative Dentistry Director Operative Dentistry Division University of TennesseeCollege of Dentistry Memphis, Tennessee | ||
| Waldemar G. de Rijk, PhD, DDS Associate Professor Department of Restorative Dentistry Director Division of Biomaterials University of Tennessee College of Dentistry Memphis, Tennessee | ||
| James F. Simon, DDS, MEd Professor Department of Restorative Dentistry Director Division of Esthetic Dentistry Director Division of Clinical Research University of TennesseeCollege of Dentistry Memphis, Tennessee | ||




 www.comsol.com/showroom/gallery/245.php
www.comsol.com/showroom/gallery/245.php