Industry Insider

LED Medical Diagnostics Inc., a Vancouver-based medical device company, recently closed a USD $6 million financing round through Integral Wealth Securities Limited.
The company will use these funds to aggressively market and distribute its FDA (510k) cleared VELscope® (Visually Enhanced Lesion Scope) oral mucosal examination device in the United States and Canadian dental markets through its wholly-owned subsidiary, LED Dental Inc.
VELscope is a clinically-proven adjunctive screening device that uses a narrow band of blue light and specialized filtering technology to help dental professionals evaluate oral mucosa for abnormal areas of concern, such as potentially cancerous lesions that may not be apparent under white light.
In addition, this round of funding will enable LED Medical to accelerate its efforts to integrate the use of VELscope into the oral hygiene and oral pathology curricula of leading dental schools throughout North America.
VELscope was proven effective in clinical trials conducted by the BC Cancer Agency (BCCA) and financed in part by over $50 million in grants from the National Institute of Dental and Craniofacial Research, part of the National Institutes of Health, a US government-funded agency.
METLIFE AND PREVISER LAUNCH ORAL HEALTH DISEASE MANAGEMENT PILOT PROGRAM
MetLife (New York, NY) and PreViser Corporation (Mount Vernon, WA), a developer of oral health risk assessment software technology, launched a new pilot program to help identify risks for periodontal disease. MetLife will provide dentists in the pilot program free access to PreViser’s Oral Health Information Suite™, an oral health risk assessment software, through the Web site portal www.metdental.com.
Participants for the pilot program are a select group of dental practices and dentists from MetLife’s Preferred Dentist Program (PDP), a nationwide network of over 95,000 dentist locations.
The PreViser oral health risk assessment software enables a dentist to input information and determine a patient’s risk score for developing periodontal disease. The tool also generates a score of the severity of the periodontal condition for those who already manifest symptoms. Knowing this information, dentists can then better develop a customized approach for preventing and/or treating periodontal disease conditions for individual patients.
Patients also receive a personalized report that provides them with actionable suggestions to better address their periodontal care needs. Enhancing the periodontal health of patients is considered essential not only for oral health, but also because clinical studies have implicated periodontal disease as a complicating factor in other serious health problems including cardiovascular disease, diabetes, and low birth weight babies.
For more information, visit www.previser.com or www.metlife.com.
GLIDEWELL LABORATORIES RE-ENTERS THE IMPLANT MARKET; USES CAD/CAM TECHNOLOGY TO CUSTOMIZE IMPLANT ABUTMENTS
Glidewell Laboratories (Newport Beach, CA) has re-entered the implant market after a 13-year hiatus. The lab has not actively promoted implant dentistry since 1993, when James R. Glidewell, president and CEO of Glidewell Laboratories, sent a letter to his entire customer list stating that he was no longer accepting implant cases.
"I had never written a letter like that before in my career," says Glidewell. "As a lab owner you never want to turn away cases or risk your relationship with customers, but, at the time, I felt that the state of implant dentistry often left the dentist and patient in a compromised position."
In 1993 the accepted method of placing implants was with screw-retained prosthetics. These were often elaborate designs having superstructures that fastened into substructures with screws that threaded into other screws. These fixed-removable restorations typically required multiple parts and several screwdrivers and wrenches, and came with a heavy price tag. To make things even more complicated, the understanding of bone biology at the time required that implants be placed where the bone was, often having little relation to where the teeth needed to be.
Advances in implant surface technology have improved healing response and reduced recovery time, and what was once a complex restorative process has given way to more friendly procedures that closely resemble common crown and bridge techniques.
Glidewell plans to incorporate CAD/CAM technology and CT-based 3-dimensional treatment planning technology into its processes to provide general dentists with real-world, immediate solutions that will help them succeed in the growing dental implant field.
ORAPHARMA, INC. TO ACQUIRE DISTRIBUTION RIGHTS TO OSSIX® PLUS
OraPharma, Inc (Warminster, Pennsylvania) is acquiring the distribution rights to OSSIX® PLUS (Resorbable Collagen Membrane), effective April 12, 2007.
OSSIX PLUS, a resorbable collagen membrane derived from porcine tendons, is currently distributed by 3i Implant Innovations, Inc (Palm Beach Gardens, FL). Beginning April 12, the product will be available from all OraPharma account managers or via OraPharma’s customer service department at 866-273-7846.
For more information on OSSIX PLUS, contact Lyn Soloway at 215-956-2200 or lsoloway@cpcus.jnj.com.

Atlantis Components, Inc. (Cambridge, MA) recently awarded Atlantis Elite™ Lab status to Ottawa Dental Laboratory, Ottawa, IL. Ottawa Dental Laboratory was founded in 1937 by Tony Caruso and today is a leader in the advancement of dental technology.
Atlantis designs and fabricates unique dental abutments that reduce lab prep tasks and clinical chair time, thus reducing costs for all members of the implant team. Atlantis provides implant abutments in titanium, gold-shaded titanium, and zirconia that need no field-based modification prior to restoration for the widest variety of implant systems in the United States. The Atlantis Elite Dental Laboratory Program provides exclusive benefits to lab customers who are committed to improving and growing their implant business with Atlantis.
For more information, contact Ottawa Dental Laboratory at 1-800-851-8239 or visit www.ottawadentallab.com to order Atlantis abutments.
DentalEZ® Announces StarDental®, KaVo,and Midwest Style Ceramic Bearing Turbines Now Available
From DentalEZ® Group (Malvern, PA), StarDental’s patented ceramic bearings are now available in KaVo Dental Corporation (Lake Zurich, IL) and Midwest style turbines.
The expanded product line offers dental professionals ceramic bearing turbine options. With a two-year warranty included on all lubricated ceramic bearing turbines, DentalEZ offers the advantage of purchasing its patented ceramic bearings in four KaVo styles and five Midwest style turbines in the most popular handpieces.
For more information on StarDental, KaVo, and Midwest style LubeFree and lubricated ceramic bearing turbines, call 1-866-DTE-INFO or visit www.dentalez.com.
SIRONA DENTAL SYSTEMS REACHES 700TH INLAB® MILESTONE
Sirona Dental Systems (Charlotte, NC) announced that it has reached yet another milestone in its North American sales of the popular inLab CAD/CAM system, following the recent installation of its 700th inLab system.
In just a few short years since its introduction, inLab® has achieved widespread acceptance in the dental laboratory market sector while meeting dental professional demands for strong, esthetic, all-ceramic CAD/CAM restorations.
Designed with the specific requirements of dental laboratories in mind, the inLab system has the ability and versatility to fabricate restorations, including copings, multiple-unit bridges, inlays, onlays, and full contour crowns from a large array of different materials. These restorations are clinically proven, strong, and esthetically pleasing.
Dr. Barry Musikant and Dr. Allan Deutsch Launch Endoblog
Dr. Barry Lee Musikant and Dr. Allan S. Deutsch, authors of more than 250 peer-reviewed clinical and academic research articles, and members of some of dentistry’s most prestigious associations, launched www.endoblog.com. Over 60 years of clinical success has enabled them to pursue their first love, the sharing and dissemination of knowledge with the rest of the dental community. This dental blog touts the latest in web technologies such as videos, podcasts, rss feeds, and so on. For more information, visit www.endoblog.com.
3D Diagnostix Introduces 24-Hour Dicom to Simplant Conversions
CT scan to Simplant conversion of dental scans is available to dental practitioners, imaging centers, and hospital radiology departments with 24-hour turnaround. 3D Diagnostic, Inc (Brighton, MA) offers overnight service priced at $100 per arch and includes clean-up of artifacts. Custom colorization of vital anatomy is $50 extra.
The conversions of conebeam CT, and MDCT scans provide axial, coronal, and sagittal panoramic and cross-sectional images of the maxilla or mandible for distance and density measurements and the virtual placement and resizing of implants and abutments.
The availability of 3-dimensional imaging for interactive visualization and planning is an essential clinical tool for surgeons and restorative dentists performing implant and maxillofacial procedures and looking for ways to improve outcomes and reduce chair time while instilling patient confidence.
For more information, call 617-820-5279 or visit www.3ddx.com
ADSTRA ADD SELECTRONIC PATIENT SIGNATURE FEATURE
ADSTRA SYSTEMS (Toronto, OT, Canada) has added the ability to capture electronic patient signatures as part of its popular Practice Made Perfect® Dental Management Software. The new feature allows dental staff to electronically record a patient’s signature on consent forms and medical conditions documents in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Practice Made Perfect now integrates with Interlink Electronics’s ePad Electronic Signature Pad, a handwritten electronic signature capture device for capturing, binding, and authenticating electronic signatures. The ePad has become the standard signature capture technology in financial services, healthcare, and Fortune 1000 enterprise applications.
The electronic signature function was added to the Medical Conditions screen, enabling the patient to electronically sign the status of his or her medical conditions. The software can also be used to create documents to be signed by the patient. Practice Makes Perfect keeps track of all signed documents in the Signed Documents Log. Staff can then print and re-print signed documents that include the patient’s signature.
For further information call 1-800-450-0220, or visit www.adstra.com.

Atlantis Components, Inc (Cambridge MA) awarded Atlantis Elite™ Lab status to Van Hook Dental Studio in Tempe, AZ. Van Hook has been nationally recognized as a premier laboratory with an emphasis on dental implants and high esthetics.
The Atlantis Elite Dental Laboratory Program provides exclusive benefits to lab customers who are committed to improving and growing their implant business with Atlantis.
To order Atlantis abutments through Van Hook Dental Studio, call 1-800-987-4665.

Chicago Mayor Richard M. Daley served as honorary chairman for Oral Health America’s (OHA), Chicago, IL, 17th Annual Gala Dinner and Silent Auction on February 22, 2007 at Navy Pier in Chicago, IL. The event raised funds to support educational and service programs designed to improve oral health.
Mayor Daley was chosen by Time Magazine in its April 25, 2005 issue as the best out of five mayors of large cities in the United States. He has presided over such successes as the resurgence in tourism, the modernization of the Chicago Transit Authority, the building of Millennium Park, increased environmental efforts, and the rapid development of the city’s north side.
The Oral Health America Gala, held each year during the Chicago Dental Society Midwinter Meeting, is one of the dental industry’s premier networking events. This year’s theme was "Connect in Chicago." For more information, contact Joe Donohue at OHA by calling 312-836-9900 or e-mail him at joe@oralhealthamerica.org.
Ultradent Implements "CustomerVoice," an Anonymous Feedback System for Customers
Ultradent Products, Inc (South Jordan, UT) added CustomerVoice, an anonymous feedback system, to its Web site. CustomerVoice provides the opportunity for customers to submit suggestions, complaints, or questions online.
Feedback on CustomerVoice is forwarded to the appropriate department or individual at Ultradent for evaluation and response. All communication remains private during the feedback process. Customers can request that they remain anonymous to Ultradent. Feedback is used to ensure continuous improvement to products, services, and to recognize best practices.
For more information about CustomerVoice or Ultradent’s online ordering system, visit www.ultradent.com (to link to CustomerVoice, click on "Feedback"), or call 1-800-552-5512.
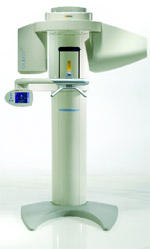
Sirona Dental Systems, Inc (Charlotte, NC) is once again expanding its relationship with its long-time partner, the UNC Chapel Hill School of Dentistry, to conduct clinical investigations of Sirona’s new 3-dimensional (3-D) imaging system, GALILEOS. The School of Dentistry, a Sirona partner for more than 15 years, will conduct research in several areas, comparing Sirona’s new 3-D technology to conventional 2-dimensional (2-D) imaging and exploring new innovative uses of 3-D technology.
Specific research areas will include the detection of carious lesions in the pits and fissures of teeth; the identification of vertical root fractures; an evaluation for implant imaging and treatment planning; and the integration of GALILEOS 3-D images with an ongoing 3-D imaging study to evaluate craniofacial surgery and orthodontic treatment.
Conventional film and 2-D digital imaging have historically proven insufficient in these functional areas.
The School of Dentistry has plans to use GALILEOS to produce images for its radiology courses. Currently most dental schools do not integrate 3-D imaging into their curricula.
Sirona will install a new imaging system at UNC prior to market release, for clinical and scientific feedback on such qualities as ease of use, diagnostic accuracy, and software functionality. UNC’s studies were published in respected dental journals, and presented at events nationwide.



