Socket Preservation Techniques
Gordon Douglass, DDS
After the extraction of teeth, there is resorption of the alveolar ridge resulting in loss of height and width.1,2 The reduction in width occurs primarily on the buccal side of the edentulous ridge, creating a potential esthetic problem for prosthetic or implant dentistry. Today, using socket preservation techniques, it is possible to preserve the height and width of the edentulous ridge. This is particularly important for placement of dental implants allowing for more ideal placement for the final esthetics with improved emergence profile and gingival architecture.
The challenge for the dentist is to preserve the quantity and quality of the gingival and osseous tissues. Additionally, the procedures used should achieve these goals but not create any significant future problems. For example, primary closure was used in many of the early attempts to preserve the alveolar ridge. Although it did help retain and protect the graft and/or barrier membrane that was placed, there often was a decrease in papillae height and keratinized gingiva.3 The techniques available today are based on the principle of guided bone regeneration, which has been used in periodontal regeneration since 1982.4 The technique consists of isolating a bony space, in this case an extraction socket with a barrier membrane, to exclude the epithelial cells and thereby have the space fill with bone. The technique has been used with and without a bone replacement graft. The placement of a barrier membrane without a bone replacement graft reduces ridge resorption.5
The use of a bone replacement graft alone results in some preservation of alveolar height and width but less than with a barrier membrane.6 The use of a barrier membrane plus a bone replacement graft has been shown to be superior to a bone graft or barrier membrane alone.7-9 The characteristics of the bone replacement graft and the barrier membrane greatly affect the final result.
The other factors that are critical for the preservation of the alveolar ridge at the time of tooth extraction are the extraction technique and the flap design. Atraumatic extraction techniques should be used with attempts to preserve all of the remaining alveolar bone adjacent to the tooth. The elevation of buccal lingual flaps, which are often needed in the extraction of badly broken-down teeth, will result in some loss of adjacent papillae height.
Barrier Membranes
The first barrier membrane used for extraction socket augmentation was expanded polytetrafluoroethylene (pTFE), which required primary closure and a second surgical procedure.7,10 This technique is still used for large augmentations of deficient edentulous ridges. The key characteristics of an ideal barrier membrane are biocompatibility, suitable occlusive (barrier) qualities, and durability upon exposure. A membrane that can be exposed eliminates the need for primary closure.11 There are numerous barrier membranes available today for a variety of guided tissue techniques. The two that have the best characteristics for extraction socket augmentation are dense pTFE and porcine collagen.
Porcine collagen membranes (Bio-Gide®, Geistlich Biomaterials, Zurich, Switzerland) have an excellent combination of characteristics. The membrane is biocompatible, resorbable, it is easy to use, it has suitable occlusive qualities, and it can be left exposed on relatively small spaces. The weakness of the membrane is a lack of rigidity, requiring support from a bone replacement graft, and one layer of the membrane may not withstand wide exposures. Therefore, two layers of the membrane should be used in the exposed area. The biocompatibility of this membrane makes it an excellent choice for use in esthetic areas where preservation of the gingival tissues is critical.
Dense pTFE is also a useful barrier membrane for extraction socket augmentations. It does not require primary closure, it has excellent occlusive qualities, and it is easy to use. The primary weakness of the membrane is that it must be removed at 4 to 5 weeks, and the placement position is critical to avoid a superficial soft tissue defect and/or a loss of papillae height. Therefore, this can be the barrier membrane of choice in the posterior where papillae height is not as critical (Figure 1; Figure 2; Figure 3 and Figure 4; Figure 5).
In badly damaged sockets, a combination of membranes can be used. For extraction sockets with complete loss of the buccal plate, a stiffer collagen membrane, such as BioMend® Extend (Zimmer Dental, Carlsbad, CA), can be placed on the buccal to assist with space maintenance, and a more flexible and exposure-resistant collagen membrane (Bio-Gide) can be placed over the socket (Figure 6; Figure 7; Figure 8; Figure 9). Dense pTFE can be used to protect any resorbable membrane from premature breakdown.
Bone Grafting Materials
The bone grafting material performs the important functions of assisting the barrier membrane in holding space and providing a biocompatible matrix for bone formation. The materials that have been used are primarily osseoconductive, providing a scaffold for bone formation. One material, demineralized freeze-dried bone allograft (DFDBA), is also somewhat osseoinductive, interacting with host cells to induce bone formation. The materials commonly used are autogenous bone, anorganic bovine bone, freeze-dried bone allograft, and beta tricalcium phosphate (bTCP), which all are osseoconductive, as well as DFDBA, which is osseoinductive.12
It is important to remember that all of these materials actually slow the rate of new bone formation, but the clinician is trading volume of bone for new vital bone.13 Studies have reported 5% to 35% residual graft materials and 30% to 60% vital bone at varying time intervals.14-16 Iasella and associates reported 58% new vital bone in untreated extraction sockets at 4 months.17
The addition of surgical-grade calcium sulfate (CaS) to autogenous grafts or to grafts of DFDBA has shown increased angiogenesis and more rapid formation of vital bone.18-20 Vance and colleagues compared a putty DFDBA plus calcium sulfate with carboxymethylcellulose to anorganic bovine hydroxyapatite (ABH) and a membrane in extraction sockets. At 4 months they reported equivalent volumes, but the CaS and DFDBA combination demonstrated 61% vital bone compared to 26% vital bone for the ABH.21 This is consistent with the findings of Guarnieri and colleagues, who reported 58.6% vital bone in extraction sockets after grafting with medical-grade calcium sulfate. Therefore, the addition of CaS to grafts (particularly DFDBA) may result in the acceleration of new vital bone formation and healing that is similar to an ungrafted socket but with increased ridge volume.
The various grafting materials can be combined to change the characteristics of the bone replacement graft. If DFDBA plus CaS is used as the basic graft, it can be made more substantial for badly damaged sockets by the addition of ABH or bTCP (BioOs, Geistlich Biomaterials; Cerasorb, Curason, Research Triangle Park, NC). For those patients who do not want a bone replacement graft from human or animal sources, bTCP is a suitable alternative that has been reported to be 60% to 70% resorbed at 6 months.
Tooth Extraction
Atraumatic extraction techniques should be used to minimize damage to the alveolar bone during tooth extraction. The initial incision can be made with a microsurgical blade to minimize elevation of the gingival tissues. The blade incises the gingival fibers and begins separation of the periodontal ligament. The periodontal ligament can then be further separated from the root with the use of a periotome, avoiding the buccal plate (such as shown in Figure 7). Frequently, single roots can be extracted with the periotome alone. If there is adequate tooth structure it can be extracted with tapered forceps, which better adapt to the root surface. For single-rooted teeth with inadequate tooth structure for forceps extraction, an alternative technique is to use the Easy-X-tractor® (A. Titan Instruments, Hamburg, NY). The Easy-X-tractor is a device designed to extract single-rooted teeth with minimal trauma to the alveolus and does not require flap elevation (Figure 10 through 12).
If the tooth structure is too broken down for any of the above techniques, the roots can be carefully sectioned into fragments and extracted without placing pressure on the alveolus. Once the tooth is extracted, the alveolus should be thoroughly debrided, removing all of the granulation tissue and irrigated with saline. The complete removal of the granulation tissue will improve the formation of new bone in the socket.
Flap Design
The extraction of a tooth results in a decrease in support of the papillae and some loss of papillae height (Figure 13). The loss of papillae height is increased with the elevation of buccal and lingual flaps.22 In areas of esthetic concern, consideration should be given to extracting the tooth and augmenting the socket without elevating a flap or the use of a mini-flap on the buccal preserving the papillae (such as shown in Figure 8).
Postoperative Management
The most important step in the postoperative management of augmented extraction sockets is the placement and shaping of the temporary tooth that is placed over the extraction area. If the socket is augmented and an immediate implant is not placed, a removable ovate pontic can be placed immediately. The pontic form can be altered during the healing period with light-cured resin to optimize the pressure on the healing tissues and direct the gingival form. The loss of papillae height from extraction can be largely restored with the use of removable or fixed provisional appliances.
CONCLUSION
The socket augmentation techniques consist of several important steps: minimal flap reflection, an atraumatic extraction, thorough debridement of the socket, placement of a bone replacement graft, the use of a barrier membrane or membranes, and stabilizing the tissues with sutures. It is important to remember that there is no one perfect technique for the augmentation of extraction sockets at the time of tooth extraction. The type of bone replacement graft used will affect the stability of the graft and the rate of new bone formation. The addition of CaS to the graft materials appears to accelerate the rate of vital bone formation. The only barrier membrane that can be predictably left uncovered with one layer is dense pTFE, but it may result in a healing pattern that may be of concern in esthetic areas. Some collagen membranes can be left exposed but are best used in two layers to prevent premature exposure of the grafted socket. Any resorbable membrane can be covered with a dense pTFE membrane to protect it from early breakdown. By combining the various graft materials for the desired features and using dual membranes, it is possible to augment any extraction socket with a predictable result.
References
1. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.2. Devlin H, Freguson MW. Alveolar ridge resorption and mandibular atrophy. A review of the role of local and systemic factors. Br Dent J. 1991;170(3):101-104.
3. Simon BJ, Von Hageen S, Deasy MJ, et al. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J Periodontol. 2000;1(1):1774-1791.
4. Nyman S, Lindhe J, Karring T, et al. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290-296.
5. Lekovic V, Camargo PM, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69(9): 1044-1049.
6. Tatum OH Jr. Osseous grafts in intra-oral sites. J Oral Implantol. 1996;22(1);51-52.
7. Buser D, Dula K. Belser U, et al. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993;13(1):29-45.
8. Seibert J, Nyman S. Localized ridge augmentation in dogs: a pilot study using membranes and hydroxyapatite. J Periodontol. 1990;61(3): 157-165.
9. Buser D, Bragger U, Lang NP, et al. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990;1(1): 22-32.
10. Dahlin C, Andersson L, Lindh A. Bone augmentation at fenestrated implants by an osteopromotive membrane technique. A controlled clinical study. Clin Oral Implants Res. 1991;2(4):159-165.
11. Kirkland G, Greenwell H, Drisko C, et al. Hard tissue ridge augmentation using a resorbable membrane and a particulate graft without complete flap closure. Int J Periodontics Restorative Dent. 2000;20(4): 382-389.
12. Boyan BD, Ranly DM, Schwartz Z. Use of growth factors to modify osteoinductivity of demineralized bone allografts: lessons for tissue engineering of bone. Dent Clin North Am. 2006;50(2):217-228.
13. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000;71(6):1015-1023.
14. Smukler h, Landi L, Setayesh R. Histomorphometric evaluation of extraction sockets and deficient alveolar ridges treated with allograft and barrier membrane: a pilot study. Int J Oral Maxillofac Implants. 1999;14(3):407-416.
15. Becker W, Urist M, Becker BE, et al. Clinical and histologic observations of sites implanted with intraoral autologous bone grafts or allografts. 15 human case reports. J Periodontol. 1996;67(10):1025-1033.
16. Froum S, Cho SC, Rosenberg E, et al. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: a pilot study. J Periodontol. 2002;73(1):94-102.
17. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7): 990-999.
18. Strocchi R, Orsini G, Iezzi G, et al. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. J Oral Implantol. 2002:28(6):273-278.
19. MacNeill SR, Cobb CM, Rapley JW, et al. In vivo comparison of synthetic osseous graft materials. A preliminary study. J Clin Periodontol. 1999;26(4): 239-245.
20. Kim CK, Chai JK, Cho KS, et al. Periodontal repair intrabony defects treated with a calcium sulfate implant and a calcium sulfate barrier. J Periodontol. 1998;69(12): 1317-1324.
21. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
22. Douglass G. Alveolar ridge preservation at tooth extraction. CDA Journal. 2005;33(3):223-231.
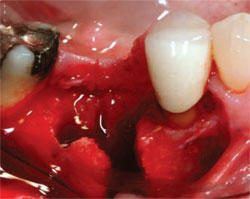 |  | |
| Figure 1 The extraction of a mandibular molar with significant loss of the buccal plate. | Figure 2 The socket is grafted with DFDBA, ABH, and CaS. | |
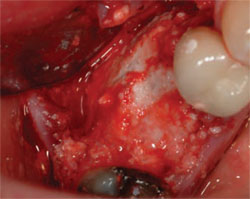
 |  | |
| Figure 3 and Figure 4 The grafted socket is covered with a dense pTFE membrane, which is left exposed. | Figure 5 The final implant restoration. | |
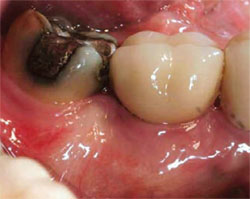
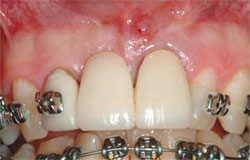 | 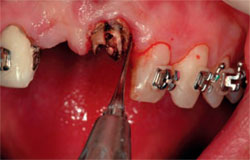 | |
| Figure 6 A central incisor with a vertical root fracture. | Figure 7 The tooth is extracted with a periotome. | |
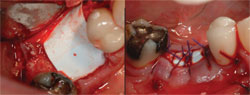
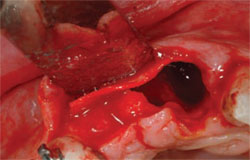 | 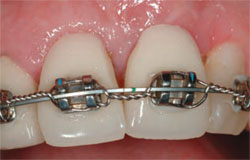 | |
| Figure 8 Only a minimal buccal flap is elevated and a firm collagen membrane is placed to add support on the buccal. | Figure 9 Healing at 3 months. | |
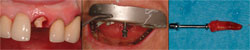 | 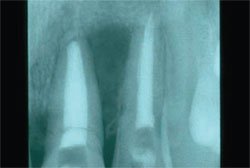 | |
| Figure 10 through Figure 12 A fractured central incisor is extracted with the Easy X-tractor® with minimal trauma to the alveolus and gingival tissues. | Figure 13 Tooth extraction results in decreased support of the papillae and loss of papillae height. | |
| About the Author | ||
| Gordon Douglass, DDS Associate Clinical Professor New York University of School of Dentistry New York, New York Private Practice Sacramento, California | ||




