You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The history of bone replacement in the human body using different materials that can be purchased, processed, or harvested dates back more than a century. Significant issues related to graft materials and growth enhancers concern their origin, rationale for use, how they are removed or eliminated from the surgical site, and their associated biologic “costs.” Another key issue when considering bone graft materials is the goal of the surgeon regarding “repair” or “regeneration” in the specific area treated.
Repair is the replacement of a part with something that is physically similar but is neither biologically nor physiologically identical to the original structure. In contrast, regeneration involves a complex series of material usage and events that enable the missing body part to be replaced by a biological structure or group of structures in all ways identical to what was lost. Surgeons contemplating replacement via either repair or regeneration should consider the options in bone replacement graft materials, growth enhancers, and barriers in terms of their expected ultimate biologic impact at the endpoint of the surgical procedure. Depending on the procedure, there may be tradeoffs between percentage of vital bone, volume augmentation, and speed of healing, versus the simplicity of the procedure.
Guided tissue regeneration involves a number of steps to achieve the goal of bone formation in an area. It requires a scaffold on which the bone is laid down. Blood vessels must enter the area to bring nutrients and the necessary cells. Calcium is important for mineralization of the organic matrix formed. Osteoblasts will deposit the collagen that will become bone. Signaling molecules assist in the attraction of the precursor cells (osteoblasts, endothelial cells) to enter the area where their specific tissues will be built. It can be daunting to complete these tasks in an area of the mouth where there is trauma from food and masticatory muscles, bacteria, and saliva—challenges that can be further complicated in a non- or poorly compliant patient. Having a better understanding of the materials and the results that can be predictably achieved with them can help the appropriately trained surgeon prepare for these surgical endeavors.
Considerations for Bone Replacement
The choices facing the surgeon at the time of extraction, ridge augmentation, or sinus graft are numerous, and are concerned mainly with the following:
Mode of entry to the site—Modes of entry may be flapless, minimally invasive, or a “conventional” large flap may be used for access.
Source of bone replacement graft materials—Autogenous bone, allogeneic bone, alloplast, xenografts, or an autogenous blood harvested/concentrated product comprise the options for bone replacement graft materials.
Type of graft used in the procedure—Grafts may be particulate, putty, or block. They are available with large or small particles, a combination of porosities, and from specific locations of origin (eg, cortical, cancellous).
Specific characteristic of each graft—There may be varying degrees of mineralization, material composition and formation, and resorbability. Also to be considered is whether the material is osteoconductive or osteoinductive and how quickly—or slowly, or if at all—the graft will resorb.
Barrier type—The barrier may be synthetic or it could be collagen—in which case its source should be considered; it may be cross-linked or processed in a “natural” way; it also may be resorbable or removable, bioactive, or inert.
Early studies on extraction therapy demonstrated that placement of neither a graft nor a barrier resulted in vital bone formation in the socket.1 While in principle, this is an outstanding result, there are concerns related to using this as the only mode of preservation of the alveolus at the time of tooth removal. Fickl et al2 in 2008 investigated the differences between flap elevation and flapless extraction, with and without bone replacement grafting, in dogs. They found that raising the mucoperiosteal tissues resulted in more loss of alveolar width and height than leaving the soft tissues intact at the time of surgery. They also noted more, though not full, preservation of the dimensions by placement of a combination bone graft with anorganic bovine bone mineral in collagen than no grafting. Iasella et al3 demonstrated that more vital bone was formed when no graft material was placed at the time of tooth extraction, but there was a more than 30% loss of ridge width than when a mineralized allograft was inserted into the defect and covered with a resorbable collagen barrier.3
In a study comparing two different grafting methods,4 where bovine-derived xenografts were placed in a socket and covered with a resorbable barrier there was a slight gain in height, but only 26% vital bone formed. When a composite graft with demineralized allograft and calcium sulfate was placed and covered with a calcium sulfate barrier, the same amount of horizontal ridge collapse occurred, but over two times the amount of vital bone was viewed histologically.
Grafting and Barrier Materials
Calcium Sulfate
Other graft materials that have been used at the time of tooth extraction include synthetic materials. Calcium sulfate has been used in many configurations as a graft and/or enhancer (Figure 1). A 2004 study showed excellent volume preservation and that close to 60% vital bone formed when a hemihydrate form of calcium sulfate was used with no barrier.5 A novel bone replacement graft material described in a 2012 report is biphasic in nature, containing both hemi- and di-hydrate calcium sulfate. This material is self-setting in the presence of blood or saliva, is reinforced, and can be used with or without a barrier membrane.6 The report presented findings on its use in multiple types of defects, all demonstrating significant amounts of bone histologically. In addition, there was significant preservation or augmentation of the alveolar ridge width.
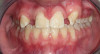
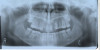
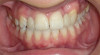
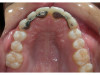
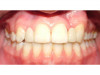
The patient shown in Figure 1 had presented after significant endodontic-related abscesses reduced the height of both buccal and lingual plates of bone. Covering the biphasic calcium sulfate (BPCS) graft with a dense polytetraflouroethylene (PTFE) barrier for 3 weeks enhanced healing in the site. Clinically, it is apparent that the ridge volume was maintained over 5 years and supported keratinized tissue (Figure 2). Most importantly, there was, on histologic evaluation, 58% vital bone, with no remnants of the bone replacement graft material (Figure 3).
Beta-Tricalcium Phosphate
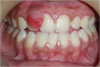
There are concerns that graft materials that fully resorb in a short timeframe may contribute to site collapse. Pure-phase beta-tricalcium phosphate (ß-TCP) (Figure 4) was one of the materials developed to address this concern. In a clinical and histologic study, extraction sockets were shown to have 91% of ridge width preserved when grafted with ß-TCP (Cerasorb®, Curasan, Inc., www.curasaninc.com) and covered with either a collagen or dense PTFE barrier.7 Dental implants were placed in these patients at 4 to 6 months postoperatively, a later time period than in the calcium sulfate studies. The patient shown in Figure 4 had only three maxillary teeth remaining, supporting an ill-fitting removable prosthesis. Due to the significant occlusal forces that were to be demanded on this single-tooth, implant-supported restoration, it was deemed advantageous to maximize the amount of vital bone in the recipient site. The area was left to heal for 7 months before flap exposure (Figure 5), implant placement in an ideal location, and graft analysis in the largest part of the defect. Analysis of the retrieved specimen showed 85% vital bone in the apical 90% of the core and a thin layer of nonresorbed ß-TCP at the crestal portion (Figure 6).
TCP Coated with PLGA
A new form of tricalcium phosphate has been developed for ease of use in a syringe system, which sets the material in the grafted site. The system is made up of one syringe containing 99% ß-TCP granules coated with poly(lactide-co-glycolide) (PLGA). This is mixed with an ampule containing BioLinker® (N-methyl-2-pyrrolidone and water) (GUIDOR® easy-graft®, Sunstar, www.GUIDOR.com) (Figure 7). A study demonstrated the biocompatibility and resorption of this material in extraction sockets.8 With its ability to resorb slowly and fully and to maintain its shape during healing, GUIDOR easy-graft is potentially an ideal bone replacement graft for defects of varying sizes, either with or without a barrier.
Figure 7 demonstrates the handling of the material as it is inserted into a large, mandibular molar extraction socket. The graft was covered with a collagen barrier, but no primary closure was either planned or obtained. The area healed uneventfully and was reentered with a flap procedure at 8 months for implant placement (Figure 8). Based on Figure 8 it is evident that much of the graft material had resorbed and was replaced with vital bone while the width of the alveolar ridge was preserved to facilitate ideal placement of an endosseous dental implant.
Barriers
In response to challenges with exposure and infection using expanded PTFE barriers, dense, nonporous barriers have been studied and shown to be successful for about 20 years. Using a dense PTFE barrier (Cytoplast™ TXT, Osteogenics, www.osteogenics.com) alone for protecting a blood clot has been shown in numerous papers to preserve alveolar ridge width to a great extent. In these cases, it was demonstrated radiographically and histologically that the former sockets were filled with vital bone suitable for osseointegration as early as 3 months post extraction in humans.9-11 In some instances, whether for the comfort of the surgeon or to support the membrane to maintain a larger alveolar bone volume, the site was filled with a graft material (as was shown in Figure 1 through Figure 3).
To obtain a different type of barrier required engineering with a number of components. A resorbable barrier was designed to be space-maintaining due to its rigid nature and space between the inner and outer layers (GUIDOR® Matrix Barrier, Sunstar). The porous nature of the surfaces, made of a combination of a citric acid ester and polylactic acid, enables tissue ingrowth to stabilize the barrier. Additionally, there is nutrient perfusion from the periosteum to the treated defect. The material has been shown to have a low incidence of inflammation and infection and complete resorbability in the time required for guided tissue regeneration in humans.12 These barrier characteristics may be quite useful to the dental surgeon in cases where osseous defects are present, either around implants or where bone regeneration is required prior to their placement.
In a case presented in Figure 9 through Figure 11, the synthetic barrier was inserted at the time of removal of a tooth with significant bone loss on the palatal aspect (Figure 9). The rigidity of the material not only enabled containment of the bone replacement graft material, but also the initial formation of the site in the desired alveolar ridge shape (Figure 10). Six weeks later, there was no evidence of inflammation and the area was healing quite well (Figure 11). Not only was the alveolar ridge back to its ideal shape at the crest, but there was also a widened zone of keratinized tissue where primary closure was not attempted over the barrier.
Putties
To overcome some of the issues associated with conventional particulate bone graft materials, some putties have been developed for multiple uses, including sockets, lateral-approach sinus grafts, and alveolar ridge augmentations. The previously mentioned BPCS and PLGA/TCP are two such materials. Another is a combination demineralized/mineralized allograft in a collagen gelatin carrier (Optecure®, Exactech, www.exac.com), a semi-rigid material for ridge augmentation. Because the material has, according to the manufacturer, tested positive for osseoinductivity, the amount of vital bone formed in these cases is significant.
The patient shown in Figure 12 presented with an alveolar ridge in the anterior mandible that was 1 mm wide starting 2 mm below the alveolar crest. The allograft putty was hydrated in sterile saline, covered with calcium sulfate (3D Bond™, Augma Biomaterials, www.augmabio.com) and an Ossix® Plus (Datum Dental Ltd, www.ossixdental.com) barrier, and primary closure was obtained. Narrow-diameter implants (ANEW®, Dentatus, www.dentatus.com) were used to assist in retention of a fixed transitional prosthesis. Four months later, dental implants were inserted, then restored another 4 months later. The clinical result is shown in Figure 13, which provides a 3-year post-loading view of the anterior mandible demonstrating the thickness and health of both the alveolar ridge and keratinized tissue.
Anorganic Bovine Bone Mineral
Early studies on lateral-approach sinus augmentation used anorganic bovine bone mineral as a graft.13 In a series of publications over many years,14-25 this xenograft was used alone, mixed with autogenous bone harvested from the patient, or mixed with growth factors from various sources. When the materials were used alone but covered with a barrier membrane covering the lateral window, there was a vital bone percentage ranging from 12% to 17% between 6 and 10 months postoperatively. Although this material has been shown to yield a high success rate when utilized in this manner, other materials and techniques have been investigated. One study demonstrated implant placement at the same time as sinus augmentation with only platelet-rich fibrin harvested from the patient as the material used to elevate the Schneiderian membrane.26 In this multi-center study, 25 patients had implants placed in 1.5 mm to 6 mm of residual maxillary bone at the same time as sinus augmentation. The resulting 50% vital bone at 6 months postoperatively enabled significantly faster restoration to function of the treated patients.
Conclusion
In their discussion of the histologic analysis of socket grafting with medical-grade calcium sulfate hemihydrate, Guarneri et al5 stated, “The goal of any grafting procedure is to achieve formation of 100% living bone tissue surrounding implants.” Depending on the size and location of the defect treated relative to adjacent sources of both progenitor cells and vasculature, this may or may not be possible. According to long-term studies on implant success rates in grafted bone, the incorporation of non-vital, non- or slowly resorbing particles may not make a difference.27 However, in studies that were done with fully resorbable materials, there appears to be a trend supporting earlier placement of dental implants than in other papers.9,26 The bone replacement graft, barrier, and growth-enhancing materials presented in this discussion should give the surgeon some guidelines. Varying surgical approaches and types of materials affect both the volume of bone formed and the quality and amount of vital bone it contains. By determining the endpoint of the procedure 3, 6, 9, or more months down the line, an appropriate choice of technique and materials can be made. In this way, patients can receive optimal therapy backed by science, literature, and predictability.
References
1. Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:33-44.
2. Fickl S, Zuhr O, Wachtel H, et al. Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. J Clin Periodontol. 2008;35(4):356-363.
3. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontal. 2003;74(7):990-999.
4. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
5. Guarnieri R, Pecora G, Fini M, et al. Medical grade calcium sulfate hemihydrate in healing of human extraction sockets: clinical and histological observations at 3 months. J Periodontol. 2004;75(6):902-908.
6. Horowitz RA, Rohrer MD, Prasad HS, et al. Enhancing extraction socket therapy with a biphasic calcium sulfate. Compend Contin Educ Dent. 2012;33(6):420-428.
7. Horowitz RA, Mazor Z, Miller RJ, et al. Clinical evaluation alveolar ridge preservation with a beta-tricalcium phosphate socket graft. Compend Contin Educ Dent. 2009;30(9):588-594.
8. Nair PN, Luder HU, Maspero FA, et al. Biocompatibility of Beta-tricalcium phosphate root replicas in porcine tooth extraction sockets - a correlative histological, ultrastructural, and x-ray microanalytical pilot study. J Biomater Appl. 2006;20(4):307-324.
9. Horowitz RA. Extraction environment enhancement: critical evaluation of early socket healing in long-term barrier-protected extraction sockets. Compend Contin Educ Dent. 2005;26(10):703-713.
10. Bartee BK, Carr JA. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J Oral Implantol. 1995;21(2):88-95.
11. Hoffmann O, Bartee BK, Beaumont C, et al. Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: a retrospective non-randomized study. J Periodontol. 2008;79(8):1355-1369.
12. Gottlow J, Lundgren D, Nyman S, et al. New attachment formation in the monkey using Guidor, a bioabsorbable GTR-device [abstract]. J Dent Res. 1992;71:297. Abstract 1535.
13. Wallace SS, Froum SJ, Cho SC, et al. Sinus augmentation utilizing anorganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: histomorphometric and clinical analyses. Int J Periodontics Restorative Dent. 2005;25(6):551-559.
14. Del Fabbro M, Wallace SS, Testori T. Long-term implant survival in the grafted maxillary sinus: a systematic review. Int J Periodontics Restorative Dent. 2013;33(6):773-783.
15. Testori T, Wallace SS, Trisi P, et al. Effect of xenograft (ABBM) particle size on vital bone formation following maxillary sinus augmentation: a multicenter, randomized, controlled, clinical histomorphometric trial. Int J Periodontics Restorative Dent. 2013;33(4):467-475.
16. Wallace SS, Tarnow DP, Froum SJ, et al. Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract. 2012;12(3 suppl):161-171.
17. Testori T, Drago L, Wallace SS, et al. Prevention and treatment of postoperative infections after sinus elevation surgery: clinical consensus and recommendations. Int J Dent. 2012;2012:365809. doi:10.1155/2012/365809.
18. Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26(1):123-131.
19. Tarnow DP, Wallace SS, Testori T, et al. Maxillary sinus augmentation using recombinant bone morphogenetic protein-2/acellular collagen sponge in combination with a mineralized bone replacement graft: a report of three cases. Int J Periodontics Restorative Dent. 2010;30(2):139-149.
20. Froum SJ, Wallace SS, Cho SC, et al. Histomorphometric comparison of a biphasic bone ceramic to anorganic bovine bone for sinus augmentation: 6- to 8-month postsurgical assessment of vital bone formation. A pilot study. Int J Periodontics Restorative Dent. 2008;28(3):273-281.
21. Testori T, Wallace SS, Del Fabbro M, et al. Repair of large sinus membrane perforations using stabilized collagen barrier membranes: surgical techniques with histologic and radiographic evidence of success. Int J Periodontics Restorative Dent. 2008;28(1):9-17.
22. Wallace SS, Mazor Z, Froum SJ, et al. Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. Int J Periodontics Restorative Dent. 2007;27(5):413-419.
23. Froum SJ, Wallace SS, Elian N, et al. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26(6):543-551.
24. Wallace SS. Maxillary sinus augmentation: evidence-based decision making with a biological surgical approach. Compend Contin Educ Dent. 2006;27(12):662-668.
25. Wallace SS, Froum SJ, Cho SC, et al. Sinus augmentation utilizing anorganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: histomorphometric and clinical analyses. Int J Periodontics Restorative Dent. 2005;25(6):551-559.
26. Mazor Z, Horowitz RA, Del Corso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol. 2009;80(12):2056-2064.
27. Cooper LF, Reside G, Raes F, et al. Immediate provisionalization of dental implants in grafted alveolar ridges in the esthetic zone: a 5-year evaluation. Int J Periodontics Restorative Dent. 2014;34(4):477-486.
DISCLOSURE
Dr. Leventis is a consultant to Sunstar Suisse SA, Etoy, Switzerland.
About the Authors
Robert A. Horowitz, DDS
Clinical Assistant Professor, Departments of Periodontics, Implant Dentistry, and
Oral Surgery,
New York University College of Dentistry,
New York, New York; Private Practice,
Periodontics and Implant Dentistry,
Scarsdale and New York, New York
Minas D. Leventis, DDS, MS, PhD
Researcher, Department of Oral & Maxillofacial Surgery, Dental School, University of Athens, Greece; Private Practice, Oral Surgery and Implant Dentistry, Athens, Greece
Michael D. Rohrer, DDS, MS
Professor and Director, Division of Oral and Maxillofacial Pathology, Director, Hard Tissue Research Laboratory, University of Minnesota School of Dentistry, Minneapolis, Minnesota
Hari S. Prasad BS, MDT
Senior Researcher, Hard Tissue Research Laboratory, University of Minnesota School of Dentistry, Minneapolis, Minnesota