A New Syringe-Delivered, Moldable, Alloplastic Bone Graft Substitute
Bone defects are treated using various sources. Autogenous bone is harvested from the patient at a donor site, eg, in the oral cavity or the iliac crest. This is the preferred solution for large defects. Autogenous bone contains living cells and factors that support bone regeneration. The grafted tissue is resorbed during the healing process and is gradually replaced by newly formed bone in a process called creeping substitution.1
When autogenous bone is placed on the alveolar ridge, volume losses of 30% to 60% have been observed over 1 year.2 Depending on the donor site and the technique, continuing problems such as loss of sensitivity and pain have been reported at the donor site in up to 55% of cases.3,4 Intraorally harvested bone chips are often contaminated with bacteria.5
Bone Graft Substitutes (BGS)
The limited availability of autogenous bone grafts and the risk of complications at the donor site have driven the development of bone graft substitutes (BGS) from an early stage. Bone graft substitutes act as an osteoconductive scaffold. They support bone formation, which starts from the wall of the defect and spreads through the bone graft substitute to the center of the defect. Bone graft substitutes also support and stabilize the surrounding structures and prevent volume loss at the site of the defect. They are classified by their origin.
Allogenic bone graft substitutes consist of processed human bone, generally from cadavers. The human material has the advantage of being available from accredited tissue banks. Allogenic bone is often available in a demineralized, freeze-dried form. Although allogenic materials yield good clinical results, they also have disadvantages that must be considered, such as the risk of disease transmission, particularly if the origin of the bone is obscure, and possible rejection reactions.6
Xenogenic bone graft substitutes are manufactured from non-human bone, such as bovine, porcine, or equine sources, and are therefore readily available. Xenogenic bone particles are known to resorb slowly and have been shown to remain embedded in newly formed bone after 11 years.7
Synthetic (alloplastic) bone graft substitutes are manufactured from mineral raw materials; their composition is precisely defined and availability is unlimited. There is no possibility of transmission of disease pathogens or rejection reactions. In general, patients do not have any ethical concerns when synthetic bone graft substitutes are used.
The most commonly used alloplastic bone graft substitutes consist of calcium phosphates, such as ß-tricalcium phosphate (ß-TCP) and hydroxyapatite (HA). Calcium phosphates have been in use in oral surgery for more than 20 years with very good results and are thoroughly scientifically documented.8 Their resorption characteristics can be controlled by the composition, ranging from fully resorbable to practically insoluble.
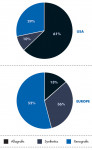
Use of the different bone graft substitutes varies worldwide from country to country (Figure 1). In the United States, allografts are preferred; in Europe, xenografts are used the most. Millenium Research9,10 attributes the highest growth rate to synthetics (Europe 9.9%, US 8.4%) due to the appearance of newly engineered products, such as GUIDOR® easy-graft® (Sunstar, www.GUIDOR.com), as well as increasing patient concerns regarding human- or animal-derived products.
Improvement Potential for BGS
Bone graft substitutes are mainly offered in the form of granules, which need to be placed in a bowl, mixed with blood or saline, transferred in several steps to the defect site, and be completely covered by a membrane to keep the granules stable in the site. This suboptimal handling procedure was the motivation to engineer a better, more user-friendly, less cumbersome product. The objective was to create a product that could be placed from the syringe directly into the defect, be moldable to the contours of the defect, and then harden to a stable scaffold. This goal was achieved by creating a novel composite biomaterial supplied in a syringe (as described below). Because of its unique easy handling properties it was named easy-graft.
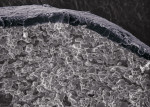
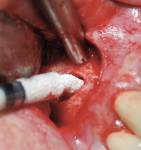
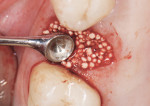
GUIDOR easy-graft products are fully synthetic and do not contain any substances of human or animal origin. The granules in the syringe consist primarily of porous calcium phosphate (ß-TCP). The unique handling of GUIDOR easy-graft is based on an extremely thin (10-μm) coating of polylactide around the granule (Figure 2). In medicine, polylactide is used to manufacture devices such as dental membranes, suture materials, resorbable screws, plates, and suture anchors for orthopedics. Before application, the granules are mixed with BioLinker® (Sunstar). This consists of water and N-methyl-2-pyrrolidone (NMP). NMP is a non-volatile solvent that has been in use in various medical products for more than 10 years. The Biolinker turns the coated granules into a sticky mass and allows moldability of the biomaterial (Figure 3), which starts to harden when in contact with blood (Figure 4).
Resorption of Graft Material and Bone Formation
The resorption process of GUIDOR easy-graft products has three stages:
N-methyl-2-pyrrolidone (NMP) is extracted within hours.
The polylactide coating (PLGA) is resorbed over a period of a few weeks.
The ß-TCP resorbs and new bone is formed over the course of several months.
In the defect, NMP is extracted by incoming blood, which leads to hardening of the material. More than 90% of the NMP is removed from the bone graft substitute within 3 hours (unpublished in-vitro data, Sunstar Degradable Solutions AG). NMP itself and its resorption products are primarily excreted through the patient’s urine within 1 to 3 days.11,12
Once in the body the polylactide coating is resorbed over 3 to 6 weeks. Throughout this period the connection between the granules becomes gradually weaker until the adhesion is completely lost. During this time, the porous biomaterial is invaded by tissue, so the material becomes stabilized with the body’s own tissue.
The resorption of the polylactide releases small amounts of lactic acid and glycolic acid. Lactic acid is found naturally in the body. It is degraded in the body by metabolic processes. Glycolic acid is a fruit acid and can be degraded in the body or excreted with the urine.
When the ß-TCP granule is exposed, it is accessible for the tissue. The larger pores between the granules provide space for the formation of new bone and for the development of blood vessels, which are required for supplying the new tissue and to remove metabolic products. Fully microporous calcium phosphates (pore size 1 µm to 10 µm) have increased osteoconductivity and increased bone formation in comparison with materials without micropores.13 Full resorption in a few months has been confirmed.14
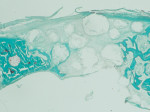
The GUIDOR easy-graft granules are openly microporous. Macropores exist in the spaces between the round, pressure-resistant granules and the interior of the particles (Figure 5 through Figure 7). The total porosity is approximately 70%. In this development of bone graft substitutes, attention was paid to ensuring that the material would not fragment or crumble during application to make sure that a comprehensive, intact pore system is retained. Figure 8 shows bone formation around the granules in a rabbit cranial model after 16 weeks.15
Unique Properties
As formulated by Hammerle and Jung in 2003: “Developments in bone augmentation procedures can be related either to simplification of the clinical handling or to influencing of biological process. To simplify clinical handling, new materials should comprise a matrix with optimal cell ingrowth capacities and good mechanical properties, providing space for tissue regeneration. No membrane and no specific procedures for mechanical fixation should be necessary. The use of synthetic (alloplastic) materials would result in lower surgical risks and lower morbidity in augmentation procedures and would represent an important step forward in simplifying bone regeneration techniques.”16
Meeting all of these criteria, GUIDOR easy-graft thus set a trend toward BGS being delivered in syringes. Launched in Switzerland following collaboration with Zürich University and then internationally in 2007, GUIDOR easy-graft has quickly established itself as a leading alloplastic material with more than 250,000 applications in Europe.
References
1. Goldberg VM, Stevenson S. The biology of bone grafts. Semin Arthroplasty. 1993;4(2):58-63.
2. Sbordone L, Toti P, Menchini-Fabris GB, et al. Volume changes of autogenous bone grafts after alveolar ridge augmentation of atrophic maxillae and mandibles. Int J Oral Maxillofac Surg. 2009;38(10):1059-1065.
3. Goulet JA, Senunas LE, De Silva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76-81.
4. Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154-160.
5. Lambrecht JT, Glaser B, Meyer J. Bacterial contamination of filtered intraoral bone chips. Int J Oral Maxillofac Surg. 2006;35(11):996-1000.
6. Sun L, Hu Y, Ning Z, Liang Z. The correlation between immune rejection and osteoinduction of allogeneic bone grafting. Chin Med J (Engl). 1998;111(9):818-822.
7. Mordenfeld A, Hallman M, Johansson CB, Albrektsson T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res. 2010;21(9):961-970.
8. LeGeros RZ, LeGeros JP. Calcium Phosphate Bioceramics: Past, Present and Future. Key Engineering Materials. 2003;240-242:3-10.
9. North American Markets for Dental Biomaterials 2012. Toronto, Canada: Millennium Research Group; September 2012.
10. European Markets for Dental Biomaterials 2013. Toronto, Canada: Millennium Research Group; March 2013.
11. World Health Organization. Concise International Chemical Assessment Document 35 N-Methyl-2-Pyrrolidone. Geneva, Switzerland: WHO; 2001.
12. Bader M, Wrbitzky R, Blaszkewicz M, van Thriel C. Human experimental exposure study on the uptake and urinary elimination of N-methyl-2-pyrrolidone (NMP) during simulated workplace conditions. Arch Toxicol. 2007;81(5):335-346.
13. Habibovic P, Yuan H, van der Valk CM, et al. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26(17):3565-3575.
14. Nair PN, Luder HU, Maspero FA, et al. Biocompatibility of Beta-tricalcium phosphate root replicas in porcine tooth extraction sockets – a correlative histological, ultrastructural, and x-ray microanalytical pilot study. J Biomater Appl. 2006;20(4):307-324.
15. Schmidlin PR, Nicholls F, Kruse A, et al. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Implants Res. 2013;24(2):149-157.
16. Hammerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003;33:36-53.
About the Author
Kurt Ruffieux, PhD
CEO, Founder, Sunstar Degradable Solutions AG, Zürich, Switzerland; inventor/developer of the GUIDOR® easy-graft® bone grafting technology