A Bioactive Dental Luting Cement—Its Retentive Properties and 3-Year Clinical Findings
ABSTRACT—A clinical validation study was conducted to determine the performance of a new bioactive dental cement (Ceramir® C&B, Doxa Dental AB) for permanent cementation. The cement is a new formulation class, which is a hybrid material comprised of calcium aluminate and glass-ionomer components. A total of 38 crowns and bridges were cemented in 17 patients; 31 of the abutment teeth were vital and seven were non-vital. Six restorations were bridges with a total of 14 abutment teeth (12 vital/two non-vital). One fixed splint comprising two abutment teeth was also included. Preparation parameters were recorded, as well as cement characteristics such as working time, setting time, seating characteristics, and ease of cement removal. Baseline data were recorded for the handling of the cement, gingival inflammation, and pre-cementation sensitivity. Post-cementation parameters included post-cementation sensitivity, gingival tissue reaction, marginal integrity, and discoloration. All patients were seen for recall examinations at 30 days and 6 months. Fifteen of 17 subjects and 13 of 17 patients were also available for subsequent comprehensive 1- and 2-year recall examination, and 13 patients were available for a 3-year recall examination. Restorations available for the 3-year recall examination included 14 single-unit full-coverage crown restorations, four three-unit bridges comprising eight abutments, and one two-unit splint. Three-year recall data yielded no loss of retention, no secondary caries, no marginal discolorations, and no subjective sensitivity. All restorations rated excellent for marginal integrity. Average visual analogue scale (VAS) score for tooth sensitivity decreased from 7.63 mm at baseline to 0.44 mm at 6-month recall, 0.20 mm at 1-year recall, and 0.00 mm at 2- and 3-year recall. Average gingival index (GI) score for gingival inflammation decreased from 0.56 at baseline to 0.11 at 6-month recall, 0.16 at 1-year recall, 0.21 at 2-year recall, and 0.07 at 3-year recall. After periodic recalls up to 3 years, Ceramir C&B thus far has performed quite favorably as a luting agent for permanent cementation of permanent restorations. In-vitro crown-coping retention studies were also conducted using this cement and various control cementation materials. Mean laboratory retentive forces measured for Ceramir C&B were comparable to other currently available luting agents for both metal and all-ceramic indirect restorative materials.
Success and long-term performance of fixed restorations depends on a range of factors1 including type and performance of luting agent,2 biocompatibility, insolubility, and resistance against degradation. All of these factors are requirements for maintaining the seal at the margins of restorations, thus preventing ingress of bacteria leading to leakage, sensitivity, and secondary decay.3 Luting agents have represented a succession of chemistries over the past century or more, including: zinc phosphate, polycarboxylate, glass ionomer, resin, resin-modified glass ionomer, and self-adhesive resin cements. Within the class of chemically bonded ceramic materials4 a new cement has been introduced comprising a hybrid of calcium aluminate and glass ionomer, Ceramir® Crown & Bridge (C&B) (Doxa Dental AB, www.ceramirus.com).
Ceramir C&B is a luting agent intended for permanent cementation of crowns and bridges, gold inlays and onlays, prefabricated metal and cast post and cores, and all-zirconia or all-alumina crowns. The cement is a water-based composition and has been demonstrated to be bioactive.4 The term “bioactivity” refers to a property of this new cement to form hydroxyapatite (HA) when immersed in-vitro in a physiological phosphate-buffered saline solution.4 Physical properties (compressive strength, film thickness, setting time),5 microleakage (dye, bacterial),6-8 and in-vitro/in-vivo biocompatibility9 have been examined with favorable results. The cement is approved for marketing in the United States in a powder-liquid, triturated capsule delivery system.
The aim of this report is to present findings and conclusions from a 3-year evaluation of the clinical performance of Ceramir C&B as a luting cement for cast high-gold alloy and noble metal porcelain-fused-to-metal (PFM) restorations, as well as in-vitro laboratory data concerning the retentive properties of this new cement formulation.
Materials and Methods
A total of 38 crowns and bridges were cemented in 17 patients (eight males and nine females, age 25 to 79 years), of which 31 were on vital teeth and seven on non-vital teeth. The study consisted of six bridges with 14 abutment teeth (12 vital/two non-vital). One fixed splint comprising two abutment teeth, both of which had been root-canal treated, was also included in the study. Twenty-three were single units (ie, crowns)—19 on vital teeth and four on non-vital teeth. Five of the fixed partial dentures (FPDs) were three-unit bridges replacing a single missing tooth. One FPD was a five-unit bridge replacing two missing teeth. Twenty-three of the cemented crown-and-bridge units involved anterior teeth (cuspid to cuspid), while 15 were on posterior teeth. The study protocol and informed consent form were approved by the Institutional Review Board at Temple University, Kornberg School of Dentistry (IRB Protocol No. 11411). All participating subjects signed an informed consent form prior to participating in this study.
The manufacturer (Doxa Dental AB, Uppsala, Sweden) provided the cement in a powder-liquid format, with the powder being supplied in pre-dosed vials while the liquid was supplied in a dropper bottle. The powder and liquid components were dispensed and mixed according to the manufacturer’s instructions. Mixing time was accomplished within 1 minute to ensure complete incorporation of all powder in the liquid, while the working time was 2 to 2.5 minutes, allowing for ample time to place the mixture in the units to be cemented. The cement parameters evaluated in the clinical study were: dispensing, mixing, working time, setting time, seating characteristics, and ease of cement removal (Table 1).
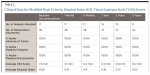
Clinical data parameters consisted of: gingival inflammation index (GI), tooth sensitivity according to both categorical and visual analogue scale (VAS)-based measurements, marginal integrity, discoloration of cement margin, and retention (Table 1). These parameters were assessed at both baseline and all recall periods. Marginal adaptation was measured clinically using the Modified Ryge/(USPHS Criteria).10 During the try-in appointment the units were assessed clinically to determine if the restorations were acceptable. The crown was again evaluated for marginal fit (designated as baseline score) using the same modified Ryge criteria after cementation. Marginal staining was assessed using the Modified Ryge criteria. Gingival response was evaluated pre- and postoperatively by means of the Loe and Silness gingival index.11
Data for VAS and GI were analyzed statistically for within-treatment cement influenced effects by comparing changes from baseline to the 1-, 2-, and 3-year recall points using the Student’s t-test for paired data (significance p < 0.05). Data for VAS were based on patient-level data, ie, the 17 patients who participated in the study. In the case of GI index (for any given evaluation time), the patient-level score represented the highest (greatest level) of inflammation scored for the restoration under evaluation.
A restoration was deemed to have failed in this clinical investigation, at any recall point, if it had:
• recurrent decay
• loss of retention
• persistent tooth sensitivity categorized as “moderate” or “severe”
• requirement for post-insertion endodontic treatment
Clinical photographs were made of selected restorations immediately following placement of the crown(s) or FPD(s) and at various recall appointments.
Laboratory measurement of the mean retentive strengths of this new cement formulation, retaining various metal and all-ceramic crown materials, was conducted using a test method described previously.12 Freshly extracted human bicuspids were positioned in the center of Teflon cylinders and imbedded in self-curing resin, leaving the crown extruded. On a jeweler’s lathe, the teeth were machined with a diamond disc under copious water cooling. The total angle of convergence measured 32 ± 1.0 and the occlusal table had a diameter of ± 4 mm.
Alternatively, in assessing the retentive strength of the cement to lithium-disilicate (e.max®, Ivoclar Vivadent Inc., www.ivoclarvivadent.com) crowns, tooth preparations with the above described parameters were fabricated in chrome-cobalt, which prior preliminary experiments indicated were representative of preparations on extracted human teeth. Copings were either prepared by the lost-wax technique and cast in type III gold alloy using routine laboratory techniques, or were machined from pre-sintered zirconia or lithium disilicate using CAD/CAM techniques. Groups of either five or 10 copings were cemented with five different cements using a pressure jig with a standardized force of pressure of 4.8 Kg. The cement was allowed to bench-set for 10 minutes, after which they were stored in sterile phosphate buffer at 37°C for 24 hours in an incubator before the copings were subjected to a tensile force in an Instron testing machine. The force at rupture was recorded in Kg force.
Results
Baseline Data
Handling characteristics of the cement have been reported elsewhere, but will be reviewed here as well to provide information regarding the baseline cementation characteristics. During the clinical investigation, mixing of the cement, cement working time, and viscosity of the cement during placement and seating were deemed to be favorable. The ease of mixing, acceptable working time (2 to 2.5 minutes), and a low, “mousse-like” viscosity were consistently observed. Clinically, it was determined that the final setting time was within 4 to 5 minutes. In all cases, try-in of restorations prior to cementation indicated complete seating of the casting with respect to fit and marginal adaptation. The favorable consistency and viscosity of the cement appeared to ensure complete seating of all castings at cementation. Removal of excess set cement from the margins was also noted to be “easy” for all subjects and restorations. No patients noted any taste, adverse or otherwise, and no patients experienced any immediate post-cementation hypersensitivity. Assessments conducted immediately after cementation indicated excellent (alpha) readings for post-cementation marginal integrity, as well as for marginal discoloration (= no evidence of marginal discolorations).
Baseline data on the key clinical performance characteristics are summarized in Table 2 and Table 3. With respect to categorical, patient-based assessment of tooth sensitivity, only seven of 17 subjects indicated some degree of pre-cementation sensitivity. With respect to pre-cementation gingival inflammation (as measured by GI scores), 16 out of 17 patients presented with GI scores of 0-1, thus indicating a low degree of gingival inflammation. Nine of these 17 patients scored baseline gingival inflammation (GI) values of 0, while seven patients scored GI values of 1. Only one patient registered a gingival index score of 2. In spite of the presence in all patients of temporary acrylic restorations cemented with temporary cements, the recorded baseline gingival inflammation levels were low. No caries lesions were recorded in any of the participating subjects. The mean pre-cementation VAS score was 7.63 ± 1.63 mm (range 0 mm to 32 mm), but only seven of 17 subjects registered a VAS score above zero. In all seven of these subjects, the positive VAS score correlated to a subjective, categorical rating for some degree of tooth/tissue sensitivity above “none.”
Summary of Findings from 1-, 6-, 12-, and 24-Month Recall Data
The detailed information regarding the performance of this luting cement up to 2 years of clinical observation has been reported elsewhere.13-15
In summary, the clinical performance appeared clinically acceptable. There were no retentive failures, and the cement appeared, from subjective clinical assessments, to be easy to use and to handle well. The tooth/soft-tissue response appeared very good and well within clinically acceptable ranges.
3-Year Recall Data
After 36 months, 11 subjects (65%) and 24 out of 37 restorations/abutments (65%) were available for clinical recall documentation. Six subjects were not available for recall at the 2- and 3-year intervals: one subject had deceased in the time period between the 12- and 24-month recall period; another subject was unavailable for recall due to relocation more than 500 miles from the study site and did not respond to a certified-return receipt letter; three subjects did not respond to either phone or mail contact to arrange a recall appointment; and, finally, one subject who did not report any dental problems of any kind was contacted but could not physically attend the 3-year recall.
None of the 11 patients available for the 3-year recall reported any tooth-tissue sensitivity. Marginal integrity and discoloration for all restorations received “excellent (alpha)” scores, indicating optimal marginal integrity and no evidence of marginal discolorations. Caries was absent in all examined restorations. Ten out of 11 patients had GI scores of 0, suggestive of very low levels or an absence of gingival inflammation. One of 11 patients scored a localized GI score of 1. Subjectively and numerically, as was reported previously concerning the clinical performance of this cement, post-cementation GI scores indicated good soft-tissue compatibility at the 3-year recall time period. Descriptive statistics for the 36-month GI score was an average of 0.07 + 0.27, which can be compared directly to the baseline pre-cementation average value of 0.56 + 0.62 and a 24-month recall value of 0.21 + 0.38. This represents approximately an 88% reduction in gingival inflammation at the 3-year recall compared to baseline gingival inflammation values. Statistical analysis (Student’s t-test for paired data) of GI scores, comparing baseline to the 36-month values (in a restoration-subject, pair-wise fashion) for the 11 recall subjects, showed a statistically significant difference between pre-cementation and 36-month data (P = 0.029, significance p < 0.05).
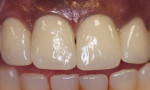
Descriptive statistics for the 36-month, post-cementation VAS scores was an average of 0.0 + 0.00, with all scores at 0 mm; all 11 subjects (at 3-year recall) registered a VAS score of zero at the 36-month recall. This VAS score represented a continued reduction over values obtained at 1-year recall (0.20 mm) and equal to the 2-year recall mean value (0.00 + 0.00). Statistical analysis using Student’s t-test for paired data indicated a statistically significant difference between the pre-cementation and 36 months post-cementation values (P = 0.03). Furthermore, in the examiner-determined, subjective, and categorical rating for the degree of tooth/tissue sensitivity, none of 11 patients were assessed as having any degree of tooth/tissue sensitivity. At the 36-month recall, none of the single-unit restorations or bridge/split abutment displayed loss of retention (100% alpha for retention). Additionally, it should be noted that up to the 3-year recall period, none of the restorations or abutments required endodontic intervention. Failure rates after 6, 12, 24, and 36 months were all 0% for both single units and FPDs. Figure 1 depicts four anterior maxillary PFM (noble alloy) crowns cemented with Ceramir C&B cement.
Laboratory Retention Data
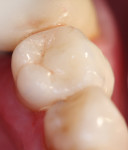
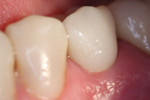
Table 4 presents in-vitro data for crown retention for Ceramir C&B and four other commercially available cements, including zinc phosphate, two different conventional glass-ionomer cements, and a self-adhesive resin cement. The data in Table 4 includes previously reported data along with more recently developed laboratory data.16 These cements were used to lute either gold alloy, zirconia, or lithium-disilicate (e.max) copings, as shown in Table 4. As can be noted in this table and using the retentive value for zinc phosphate cement of 13.9 + 4.5 as a reference standard, the mean retentive forces for Ceramir C&B ranged from 38.3 + 8.5 for gold alloy crowns, 32.1 + 6.3 for machined zirconia crowns, and 29.48 + 9.99 for the machined lithium-disilicate crowns. All mean retention values are more than double the value obtained for zinc phosphate cement luting gold alloy castings (gold alloy = 175.5%; zirconia = 130.9%; e.max = 112.1%). For zirconia crown-copings, Ceramir C&B was directly comparable to RelyX™ Unicem (3M ESPE, www.3MESPE.com), a self-adhesive resin cement, for the retentive force values (Ceramir C&B = 32.1 + 6.3; RelyX Unicem 27.8 + 11.3). Ceramir C&B was also directly comparable to Vivaglass® (Ivoclar Vivadent Inc.), a glass-ionomer cement, for the retentive force values for e.max lithium-disilicate crown copings (Ceramir C&B = 29.48 + 9.99; Vivaglass = 27.7 + 12.73). An e.max crown cemented with Ceramir C&B to a lower left second premolar is depicted in Figure 2 and Figure 3.
Discussion
A wide range of cements with a variety of chemistries are available to dental clinicians for permanent cementation of fixed restorations.17 However, because retention failures are still a major complication in fixed prosthodontics18 there is a need for further development of new and better luting cements.
There is also a desire to improve pulpal compatibility in luting cements.19 A number of the presently available cement categories have undergone systematic clinical evaluations. Pameijer,20 for example, investigated the comparative performance of zinc phosphate, conventional glass ionomer, and a urethane dimethacrylate-based resin cement over a 4-year period and found comparable clinical performance by all of them. Jokstad and Mjor21 also demonstrated successful and equivalent performance of glass-ionomer cements with that of zinc phosphate cement. The resin-modified glass-ionomer (RMGI) cements developed in the 1990s have also demonstrated acceptable clinical efficacy,22 and Jokstad23 noted that a RMGI luting cement was at least as good as zinc phosphate cement. Most recently, self-adhesive resin cements have expanded in popularity and have been evaluated for clinical performance.24 Summing up, many early clinical investigations have concluded that acid-base reaction cements, such as zinc phosphate and glass ionomer, perform well over long-term periods,20,21,23 and other more recent studies conclude that newer cement chemistries, such as resin-modified glass ionomers, resin cements, and self-adhesive resin cements, also appear to display acceptable clinical performance. In many instances, however, the time periods of clinical observation have been short.22,24,25
The introduction of any new cement chemistry prudently necessitates assessment of its laboratory and clinical performance. The laboratory performance of this new cement has been assessed with respect to a number of performance criteria. Assessment of compressive strength, film thickness, and setting time all conformed favorably to the International Standard Organization (ISO) standard for water-based luting cements.5 Comparative in-vitro microleakage performance of this new bioactive cement has also been assessed by two different methodologies. Dye leakage analysis in cemented crowns included that XeraCem (the initial name for Ceramir C&B) demonstrated significantly less leakage than a conventional glass-ionomer cement, Ketac™ Cem (3M ESPE).6 An in-vitro, bacterial leakage model comparison of Ceramir (CM) to a conventional glass-ionomer luting cement (Ketac Cem, KC) and a resin-modified glass-ionomer cement (RelyX Luting Plus, RX) demonstrated that the groups cemented with CM and RX showed no significant difference in microleakage patterns (P > 0.05), while both recorded significantly lower microleakage scores (P < 0.05) than the group cemented with KC.7,8
Biocompatibility ranks as one of the most important properties of a final luting cement; as such, a number of in-vitro and in-vivo tests (as recommended by ANSI/ADA Spec. 41 and ISO 10993) were conducted (prior to the clinical investigation) to evaluate the biocompatibility of Ceramir C&B cement.9 Results for the Ames test for mutagenicity indicated that this new cement formulation did not induce gene mutations. In-vitro cytotoxicity testing indicated cell responses ranging from none to mildly cytotoxic—an acceptable response. The skin sensitization test (in guinea pigs) indicated that this cement is not a skin sensitizer, while testing for mucous membrane irritation (hamster pouch test) indicated that Ceramir C&B produced no local irritation.9 Pulpal testing in Rhesus macaques, according to ANSI/ADA Spec. 41, indicated a virtual absence of pulpal inflammation, at both 30 and 85 days evaluation periods, after Ceramir was used to cement composite resin inlays in Class V preparations.9
Retention is perhaps the most critical factor in the performance of a final luting cement. A comparative, in-vitro crown retention study was conducted prior to this clinical evaluation to assess the retentive properties of this new cement.16 This laboratory retention study was expanded in this investigation to include two types of all-ceramic crown and bridge materials. Results of all this laboratory testing indicated that Ceramir C&B demonstrated retentive values equivalent (no statistically significant difference) to that of a self-adhesive resin cement, RelyX Unicem, yet significantly higher than a conventional glass ionomer (Ketac Cem) and zinc phosphate cement using gold alloy copings. All mean retention values for Ceramir C&B-retaining gold alloy, zirconia, or e.max copings were more than double the value obtained for zinc phosphate cement. For zirconia crown-copings, Ceramir C&B was directly comparable to RelyX Unicem, a self-adhesive resin cement. Ceramir C&B was also directly comparable to Vivaglass, a glass-ionomer cement, for the retentive force values for e.max lithium-disilicate crown copings. Clearly, these in-vitro retentive values for the experimental calcium aluminate-glass-ionomer cement correlate quite well with the clinical study findings of no retentive failures during the course of the clinical trial of this cement.
The objective of this study was to provide initial data regarding the clinical performance of a new and novel cement chemistry for the intended use of permanent cementation of high noble all-metal and PFM restorations. Systematic evaluation included assessment of specific clinical performance criteria starting at pre-cementation baseline levels compared directly to post-cementation data. Based on the available literature, this clinical report is the first to document the clinical performance of a “bioactive” cement as a luting agent for permanent dental restorations over a prolonged clinical use period (3 years). The tested water-based cement comprises calcium aluminate and glass-ionomer components.4 The dispensing, mixing, handling, and work-time/set-time properties of the cement were considered, as was its clinical performance up to 3 years.
In this study it was observed that the mixing of the cement resulted in a smooth, creamy mix, and working time (2 to 2.5 min.) was at least comparable and perhaps superior to other available cements, such as zinc phosphate, resin-modified glass ionomer, and self-adhesive resin cements. As the recorded final setting time was 4 to 5 minutes and because there was no evidence of premature setting or viscosity build-up, no difficulty was observed in the complete seating of the restorations during placement. Clean-up for the cement was also rated as “easy” and cement removal was found to be somewhat similar to that of RMGIs, ie, a distinct gel consistency formed within a few minutes, permitting easy removal. In contrast to RMGI and self-adhesive resin cements, Ceramir C&B does not, however, form an oxygen-inhibited layer on its surface, which makes clean-up much easier. Furthermore, there was no need to rush the removal, as can be the case with resin-based luting cements, which can snap-set to a hard consistency, making early removal mandatory.20
Gingival soft-tissue response improved from pre-cementation to post-cementation levels based on statistical analysis of gingival index (GI) scores. Mean gingival inflammation (GI) scores reduced to statistically significant levels below the mean baseline level for all post-cementation observation periods up to the 3-year recall. Soft-tissue response to this new cement chemistry appeared to be good and certainly within clinically acceptable levels both initially and through the 3-year observation period. As depicted in Figure 1, one can conclude that gingival health around the studied restorations improved over the course of observation after cementation. This can be attributed to the basic properties of the cement (pH = 8) within hours post hardening.
Postoperative sensitivity is a fairly common early complication in fixed prosthodontics.26 This factor was measured both qualitatively and quantitatively and demonstrated that the situation improved from baseline levels up to the 3-year recall time evaluation. After some initial sensitivity due to occlusal prematurities in the final restorations or to an incident of exposed, sensitive root dentin gingival to the marginal finish line of the cemented final restoration, and after minimal clinical intervention, there was a complete absence of categorical, subjective postoperative pain associated with any of the restorations cemented with Ceramir C&B. With respect to the correlation between subjective, patient-elicited sensitivity and VAS scores at the 3-year evaluation, none of the subjects gave a VAS score greater than zero while at the same time provided a response indicating an absence of subjective sensitivity concerning the experimental cement. Anecdotally one subject, with Ceramir C&B-cemented PFMs on the right maxillary cuspid, right lateral incisor, right central incisor, and left maxillary first premolar, also had a PFM on the left central maxillary incisor cemented with a RMGI cement. Upon questioning the subject regarding this localized, study-unrelated area at the 1-year recall, this subject localized “sensitivity when touched” to the left central maxillary incisor cemented with the RGMI. In view of these findings, and representative of the VAS scores represented in Table 3, it is reasonable to state that over the 36-month observation period the tested cement appeared to perform in a clinically acceptable fashion with respect to pulpal biocompatibility and tooth sensitivity.
There were no retentive failures of any units examined through the 3-year recall. As the degree of taper of all prepared teeth was subjectively noted and recorded for each subject, it should be noted that the majority of preparations were described as having normal, non-excessive taper. Although a small number of preparations were described as having a greater than the preferred preparation taper, none of these teeth experienced failure to this point. In an analogous fashion, surveyed and prepared crowns and abutments for acceptance of removable partial dentures were not excluded from this study. As such, none of the crowns that served as a retainer for a removable partial denture have experienced any untoward retentive effects. While this study did not intend to compare the performance of this new cement with that of any other currently available cement, it is interesting to note that one subject lost retention for a contra-lateral PFM cemented with a RMGI cement during the course of study, approximately 1 month after cementation.
While the apparent longevity and stability of zinc phosphate cement is still viewed as a “gold” standard in its use as a dental luting cement,20,21,23,24 dental cements for luting and retention of fixed dental restorations have undergone significant compositional changes over the past 50 years. Long-term success after cementation of indirect restorations depends on retention as well as maintenance of the integrity of the marginal seal. A marginal seal can be established through bonding/adhesive techniques or other mechanisms. The introduction of chemical adhesion (as in polycarboxylate and glass-ionomer cements) has been one approach to improving the performance of dental luting cements. Likewise, the inherent presence or intentional inclusion of fluoride into many of these cement formulas may provide some degree of protection in the event of material breakdown or disintegration of the cement; yet there exists only limited data to support such a protective mechanism in glass-ionomer cement.27 As such, the pursuit of alternative mechanisms to protect the cemented marginal areas of fixed restorations is worthy of consideration. Ceramir C&B, as tested here, introduces another possible functionality to the capabilities of dental cements, namely, bioactivity. When this new cement is immersed in-vitro in a physiological phosphate-buffered saline solution, hydroxyapatite (HA) is formed.4 The formation of HA, which appeared after 7 days, demonstrates that Ceramir C&B quite possibly possesses dynamic self-sealing properties, while it is speculated that actual remineralization at the margins may take place. Such a protective mechanism could provide a more durable seal of the tooth-cement-restoration interface. Furthermore, areas of marginal breakdown over time may potentially be able to be addressed through bioactivity and a resealing via deposition of HA. Additional research will be necessary to conclusively demonstrate this exciting potential capability.
To summarize, in up to 3 years of periodic clinical evaluation, the clinical performance of Ceramir C&B appears clinically acceptable. Thus far there have been no retentive failures reported or presented during recall examination. The cement appeared, from a subjective clinical assessment, to be easy to use and handle. Postoperative gingival inflammation measured both qualitatively and quantitatively improved statistically from baseline levels up to the 1-year recall time period, and remained at a lower level compared to baseline values at the 3-year recall. Postoperative sensitivity measured both qualitatively and quantitatively improved from baseline levels up to the 2-year observation point as well. As a quantitative measure of tooth sensitivity, visual analogue scores (VAS) were significantly reduced at the 6-month, 1-, 2-, and 3-year recall points, while demonstrating a continual decrease in values from 1 month through 3 years of measurement. All restorations also scored “excellent” for marginal integrity. Caries or recurrent caries was not an issue at any time during the study. All subjects tolerated the cement well, and there was an absence of any cement-related adverse events.
Conclusions
After 3 years of clinical observation, Ceramir C&B performed well and in a clinically acceptable fashion as a luting agent for permanent cementation of all-metal (high noble metal) and ceramic-fused-to-metal (noble metal) crowns and fixed partial dentures.
Laboratory retention measured through a clinically simulated tensile test method demonstrated equivalent or superior retentive force values for this cement, using zinc phosphate or conventional glass-ionomer cement as a control reference cement to lute both metal-based and all-ceramic substrate crown copings.
In conclusion, the results of this clinical evaluation combined with the extensive body of laboratory and biocompatibility data clearly indicates that this new cement chemistry appears quite acceptable for its stated clinical uses, and its bioactive properties may offer promising and new clinical advantages for the future.
Acknowledgment
This study was supported by Doxa Dental AB.
References
1. Drago C. Clinical and laboratory parameters in fixed prosthodontic treatment. J Prosthet Dent. 1996;76(3):233-238.
2. Black SM, Charlton G. Survival of crowns and bridges related to luting cements. Restorative Dent. 1990;6(3):26-30.
3. White SN, Furuichi R, Kyomen SM. Microleakage through dentin after crown cementation. J Endod. 1995;21(1):9-12.
4. Lööf J, Svahn F, Jarmar T, et al. A comparative study of the bioactivity of three materials for dental applications. Dent Mater. 2008;24(5):653-659.
5. Jefferies SR, Lööf J, Pameijer CH, et al. Physical Properties of XeraCem™. J Dent Res. 2008;87(B):3100.
6. Pameijer CH, Jefferies S, Lööf J, Hermansson L. Microleakage evaluation of XeraCem™ in cemented crowns. J Dent Res. 2008;87(B):3098.
7. Pameijer CH, Zmener O, Garcia-Godoy F, Alvarez-Serrano JS. Sealing of XeraCem™, and controls using a bacterial leakage model. J Dent Res. 2009;88(A):3145.
8. Pameijer CH, Zmener O, Alvarez Serrano S, Garcia-Godoy F. Sealing properties of a calcium aluminate luting agent. Am J Dent. 2010;23(2):121-124.
9. Pameijer CH, Jefferies SR, Lööf J, et al. In vitro and in vivo biocompatibility tests of XeraCem™. J Dent Res. 2008;87(B):3097.
10. Cvar JF, Ryge G. Criteria for the clinical evaluation of dental restorative materials. San Francisco, CA: US Department of Health, Education and Welfare, Public Health Services, US Government Printing Office; 1971. Publication No. 790-244.
11. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533-551.
12. Pameijer CH, Hultén J, Glantz PO, Randow K. Influence of low-viscosity liners on the retention of three luting materials. Int J Periodontics Restorative Dent. 1992;12(3):195-205.
13. Jefferies SR, Appleby D, Boston D, et al. Clinical performance of a bioactive dental luting cement—a prospective clinical pilot study. J Clin Dent. 2009;20(7):231-237.
14. Jefferies SR, Pameijer CH, Appleby D, et al. One year clinical performance and post-operative sensitivity of a bioactive dental luting cement—a prospective clinical study. Swed Dent J. 2009;33(4):193-199.
15. Jefferies SR, Pameijer CH, Appleby DC, et al. Prospective observation of a new bioactive luting cement: 2-year follow-up. J Prosthodont. 2012;21(1):33-41.
16. Pameijer CH, Jefferies SR, Lööf J, Hermansson L. A comparative crown retention test using XeraCem™. J Dent Res. 2008;87(B):3099.
17. Hill EE. Dental cements for definitive luting: a review and practical clinical considerations. Dent Clin North Am. 2007;51(3):643-658.
18. Burke FJ, Lucarotti PS: Re-intervention on crowns: what comes next? J Dent. 2009;37(1):25-30.
19. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications in fixed prosthodontics. J Prosthet Dent. 2003:90(1):31-41.
20. Pameijer CH, Nilner K. Long term clinical evaluation of three luting materials. Swed Dent J. 1994:18(1-2):59-67.
21. Jokstad A, Mjör IA. Ten years’ clinical evaluation of three luting cements. J Dent. 1996;24(5):309-315.
22. Yoneda S, Morigami M, Sugizaki J, Yamada T. Short-term clinical evaluation of a resin-modified glass-ionomer luting cement. Quintessence Int. 2005;36(1):49-53.
23. Jokstad A. A split-mouth randomized clinical trial of single crowns retained with resin-modified glass-ionomer and zinc phosphate luting cements. Int J Prosthodont. 2004;17(4):411-416.
24. Behr M, Rosentritt M, Wimmer J, et al. Self-adhesive resin cement versus zinc phosphate luting material: a prospective clinical trial begun 2003. Dent Mater. 2009;25(5):601-604.
25. van Dijken JW. Resin-modified glass ionomer cement and self-cured resin composite luted ceramic inlays. A 5-year clinical evaluation. Dent Mater. 2003;19(7):670-674.
26. Kern M, Kleimeier B, Schaller HG, Strub JR. Clinical comparison of postoperative sensitivity for a glass ionomer and a zinc phosphate luting cement. J Prosthet Dent. 1996;75(2):159-162.
27. Muzynski BL, Greener E, Jameson L, Malone WF. Fluoride release from glass ionomers used as luting agents. J Prosthet Dent. 1988;60(1):41-44.
About the Authors
Steven R. Jefferies, MS, DDS, PhD
Professor
Department of Restorative Dentistry
Director of Clinical Research
Kornberg School of Dentistry
Temple University
Philadelphia, Pennsylvania
Cornelis H. Pameijer, DMD, DSc, PhD
Professor Emeritus
University of Connecticut
Farmington, Connecticut
David C. Appleby, DMD, MScD, FACP
Professor Emeritus
Kornberg School of Dentistry
Temple University
Philadelphia, Pennsylvania
Clinical Professor of Restorative Dentistry
Department of Preventive and Restorative Sciences
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
Daniel Boston, DMD
Associate Dean of Clinical Affairs
Kornberg School of Dentistry
Temple University
Philadelphia, Pennsylvania
Jesper Lööf, PhD
Director of Research
Doxa Dental AB
Uppsala, Sweden