Salvaging Implants With an Nd:YAG Laser: A Novel Approach to a Growing Problem
Jon B. Suzuki, DDS, PhD, MBA
Abstract
As the incidence of implant placement expands, so too does the occurrence of implant-related pathological conditions such as peri-implant mucositis and peri-implantitis. This article will discuss implementation of a laser protocol that serves as a treatment modality designed specifically to help save ailing and failing implants. This multi-stage approach incorporates application of laser energy from a particular Nd:YAG laser with variable pulse width. This laser wavelength has demonstrated a variety of capabilities that may contribute to its clinical effectiveness. Representative long-term results of this treatment method are described, and a case depicting the protocol is presented.
The use of dental implants for the replacement of missing teeth or entire dentitions is becoming the standard of care in treatment planning for patients.1-3 This paradigm shift from fixed or removable prostheses to implants is largely patient-driven. Greater chewing efficiency, patient comfort and satisfaction, and predictable results with improved stability, retention, and function are some of the forces responsible for this shift.4,5 However, along with scientific and technological advances have come increased challenges regarding dental anatomic sites for dental implant surgical placement.
Even with 90% to 98% reported success for implants,6-8 clinical observations of implant failures are increasing. Implant failures begin initially as peri-implant mucositis and develop into peri-implantitis. Multiple or even multifactorial etiologies have been reported in the peer-reviewed literature as causes of implant failures.9 Determination of the cause(s) of implant failure dictates appropriate treatment plans for implant rescue.
Peri-implantitis, loss of attachment, and loss of osseointegration do not have well-documented therapies for management, other than case reports. This article offers a treatment option for regeneration of lost attachment and osseointegration in a peri-implantitis clinical case that is representative of many successful cases.
Minimally Invasive Protocol
The well-defined protocol (LAPIP™, Millennium Dental Technologies, www.lanap.com) provides a minimally invasive surgical methodology to address and effectively treat the growing incidence of peri-implant mucositis and peri-implantitis.10 A critical component of this protocol is the use of a specific 6.0-watt, free-running digitally pulsed neodymium:yttrium-aluminum-garnet (Nd:YAG) laser (PerioLase® MVP-7™, Millennium Dental Technologies) of 1064-nanometer (nm) wavelength with variable pulse width that is designed specifically for the treatment of periodontal disease and the LAPIP therapy.
Briefly, this multi-step protocol includes the following procedures: Surgical probings are performed under local anesthesia to record the depths of all bony defects in the soft tissue around the implant. Pocket depth determines the amount of laser energy to be delivered during the initial ablation and subsequent hemostasis applications. The laser fiber is then inserted into the periodontal pocket, oriented in a prescribed fashion, and the laser is activated at particular settings to ablate (remove) the diseased epithelial lining and granulomatous tissue and to reduce bacteria. Ultrasonic scalers and special hand instrumentation are then used to remove foreign substances (including calculus and cement) from the implant surfaces. Next, bone is modified and decorticated in a prescribed manner to stimulate the release of fresh blood and growth factors from the bone. The laser is then used again at specifically adjusted settings in hemostasis mode to form a thermal gelatinous clot containing growth factors from the bone. Coronal soft tissue is approximated against the implant using finger pressure to achieve adhesion, and then occlusal adjustment is performed to reduce traumatic forces and mobility. Splinting reduces mobility and traumatic occlusal forces.
Collectively, these procedures provide the environment for reosseointegration of the implant.
The effectiveness of the Nd:YAG laser wavelength in achieving its successful clinical outcomes under this protocol may be attributed to a variety of factors as discussed below.
Selective Photothermolysis
The first aspect is the minimally invasive, tissue-conserving quality of this laser wavelength when it is emitted at particular laser operating parameters, including power densities, energy densities, spot sizes, peak powers, pulse frequencies, and variable pulse durations. In their light microscopy study, Gold and Vilardi observed the ability of the pulsed Nd:YAG laser to effectively remove periodontal pocket lining epithelium in humans with moderate periodontitis without causing damage (necrosis or carbonization) to the underlying connective tissue.11
Ting et al made similar observations in human patients with chronic periodontitis of moderate severity treated with a pulsed Nd:YAG laser. Histologic and scanning electron microscopic examinations of tissue specimens showed laser irradiation was able to remove inflamed pocket epithelium without damage to healthy, uninflamed pocket epithelium and with no significant damage to the connective tissues. They also noted a complete absence of microorganisms in the laser-treated test specimens.12
This apparent selective photothermolytic capability of the Nd:YAG laser wavelength was demonstrated in a laboratory study by Harris and Yessik, who examined the ablation of the periodontal pathogen Porphyromonas gingivalis grown on blood agar. This medium was used as an approximation of gingival tissue. At particular settings, the laser was able to ablate the subject pathogen without visible effect on the blood agar. The researchers concluded that in a clinical setting, the pulsed Nd:YAG laser may selectively destroy pigmented pathogens while leaving the surrounding tissue intact. The pulsed 810-nm diode did not exhibit the same selectivity.13
Bactericidal Capability
A second factor is the demonstrated ability of the Nd:YAG laser to reduce pathogenic microorganisms in the periodontal pocket. In 1992 Cobb et al detected a substantial suppression of three putative microbial pathogens (Aggregatibacter actinomycetemcomitans, P gingivalis, and P intermedia) within the laser-treated pockets of human patients compared to untreated control sites. The investigators indicated their results are suggestive of a potential specific use of the Nd:YAG laser for the removal of bacteria from infected pockets.14
Twenty-two years later, McCawley (one of the members of the 1992 Cobb investigative team) and colleagues assessed the immediate post-treatment effects of Nd:YAG laser irradiation on putative bacterial pathogens in deep periodontal pockets of 20 human patients. They reported the laser immediately suppressed the tested pathogens below culture detection limits.15
Similarly, De Andrade et al found that Nd:YAG laser associated with conventional scaling and root planing produced a greater reduction of total bacteria in class II furcations of patients immediately after treatment, compared to sites that were conventionally treated.16
Giannelli and colleagues tested four different lasers and demonstrated that the Nd:YAG and 810-nm diode lasers were able to significantly reduce six periodontopathogenic bacterial species within gingival epithelial cells outside the periodontal pockets of adult patients with chronic periodontitis, without damage to connective tissue. They indicated the accompanying microvessel constriction can prevent bleeding and reduce the chances of systemic bacterial spreading during treatment.17
Relating the antibacterial effect of the Nd:YAG laser to implants, Giannini et al in their in vitro research found that specific parameters of Nd:YAG laser irradiation were able to ablate aerobic and anaerobic microbial species that were streaked on implants without damaging the titanium surface.18 Similarly, Gonçalves and colleagues showed different levels of bacterial-level reduction on the surfaces of laboratory implants by Nd:YAG and 980-nm diode lasers, depending on the settings used and the type of implant surface. Scanning electron microscopic examination revealed no implant surface changes from the irradiation.19
That the Nd:YAG laser can be used to help decontaminate implant surfaces without apparent damage to the implant itself is an important consideration for effective clinical usage. Research shows that the Nd:YAG laser—or diode, erbium, or CO2 lasers—can adversely alter implant surfaces and/or result in inadvisable temperature increases, depending on the laser settings and exposure durations selected.20-23 Therefore, laser-assisted peri-implant therapy is not recommended for a novice user. Attaining the proper qualifications through studied participation in a rigorous authorized training program is an absolute requirement.
Judicious use of the proper laser parameters, informed clinical technique, and live patient training can help achieve the results reported in a human patient study of Nd:YAG laser treatment of failing implants, performed by 21 dentists trained in the LAPIP technique. Post-treatment radiographs ranged from 2 to 48 months. All examined cases provided evidence of increase in crestal bone mass around the implant and, when reported, probe depth reductions. All clinicians noted control of peri-implantitis, reversal of bone loss, and rescue of the incumbent implant.10
Anti-Inflammatory Efficacy
The Nd:YAG laser’s antibacterial capability is related to its anti-inflammatory effect. In a study comprising 30 patients with chronic periodontitis, Gómez et al analyzed the gingival crevicular fluid samples for immunological markers of inflammation—interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α), two cytokines whose increased production in the host causes damage to soft tissues and periodontal bone support. Compared to scaling and root planing (SRP) only, the SRP-plus-Nd:YAG laser treatment sites exhibited gingival crevicular fluid with significantly lower IL-1β and TNF-α.24
The Qadri group examined 30 adult patients with periodontal pockets of 4 mm to 8 mm in a split-mouth, controlled clinical trial. Randomly assigned sites received either SRP with Nd:YAG laser treatment (test sites) or SRP only (control sites). One week after treatment, compared to the control sites, the test sites showed significant reductions in IL-1β and matrix-metalloproteinase-8 (MMP-8), the major collagenase species in inflamed human periodontium.25
Giannelli and associates evaluated the in vitro effects of Nd:YAG laser irradiation on the inflammatory response to bacterial lipopolysaccharide (LPS)-coated titanium disks. They noted that laser irradiation significantly reduced LPS-induced nitric oxide production and interleukin-8 production by endothelial cells, leading them to conclude that the use of the Nd:YAG laser may be a promising tool for therapeutically managing peri-implantitis.26
Biostimulation Effects
It may be possible that certain levels of Nd:YAG laser irradiation can stimulate aveolar bone growth at the cellular level. Arisu et al evaluated the in vitro effects of pulsed Nd:YAG laser energy at different energy settings on human osteoblast-like cell cultures. Standard colorimetric assays were used to estimate cell viability and proliferation after 7-, 14-, and 21-day incubation periods. They found that certain laser settings stimulated cell viability and proliferation while higher settings had an inhibitory effect.27
The Chellini team investigated the in vitro effects of Nd:YAG laser irradiation on osteoblasts, endothelial cells, and fibroblasts—cells representative of the oral macroenvironment. They observed significant induction in the expression of osteopontin, alkaline phosphatase, and Runt-related transcription factor 2 in osteoblasts, type I collagen in fibroblasts, and vinculin in endothelial cells, underlying molecular mechanisms demonstrative of a biostimulatory effect.28
Kim et al examined the effect of a Q-switched Nd:YAG laser (with the same wavelength as a free-running pulsed Nd:YAG laser but with higher peak power) on preosteoblast cells exposed to laser irradiation. Their in vitro results showed that high-power, low-level Nd:YAG laser energy not only increased osteoblast activity, but efficiently accelerated mineral deposition.29
Another Kim group also used a Q-switched Nd:YAG laser to investigate in vivo the capacity of bone repair in bilateral calvarial defects in rats and rabbits. They noted that periodic application of laser irradiation significantly increased new bone formation as evaluated by micro-computed tomography-based analysis and histological observation 4 weeks after surgery.30
Clinical Case
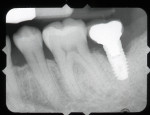
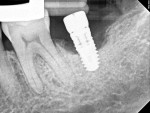
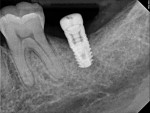
A 64-year-old female patient experienced early loss of attachment due to peri-implantitis as seen in a July 2012 periapical radiograph of an implant placed at the No. 18 site (Figure 1). Fifteen months later examination revealed marked progression of attachment loss and increase in probing depth (Figure 2).
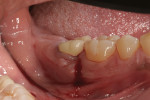
The patient presented in October 2013 with a periodontal abscess with bleeding and suppuration. Pocket depths were 12 mm on the mesial and 10 mm on the distal of the implant (Figure 3). After the primary care dentist had removed the crown, in early December 2013 the treating clinician placed a healing abutment after debriding the lesion.
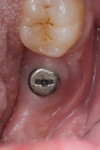
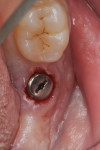
Two weeks later, on December 16, 2013, the soft tissue showed marked improvement but was still edematous (Figure 4). During this same appointment, a periapical radiograph was taken (Figure 5) and the site was treated with the LAPIP protocol (Figure 6).
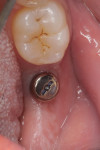
When the patient returned for post-treatment evaluation in early January 2014, significant healing had occurred and the soft tissue showed a substantially improved response (Figure 7).
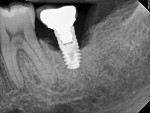
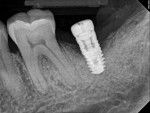
Four months after treatment, the periapical radiograph revealed the progress of the apparent bone regeneration around the implant (Figure 8).
One year after the single LAPIP treatment was completed, the periapical radiograph showed significant and progressive regeneration of peri-implant bone (Figure 9). Probing depths were reduced to 4 mm. (Author note: This case was provided courtesy of I. Stephen Brown, DDS, FACD, FICD.)
Conclusion
The aforementioned laser-related factors provide insight into the possible contributing reasons for the demonstrated clinical success of the described protocol. It should be emphasized, however, that this protocol is a multi-step approach that utilizes both conventional instrumentation and a specific Nd:YAG laser with multi-variable pulse width and specific energy settings, energy densities, pulse frequencies, peak powers, and pulse durations to achieve its results. Additional investigations will elucidate how the combination of steps performed during this procedure optimizes patient outcomes.
Armed with the proper training and carefully adhering to the prescribed step-by-step approach, general practitioners and specialists alike have an effective therapy regimen that is designed specifically to treat peri-implantitis and save ailing and failing implants.
Disclosure
The author is currently chairman of the US Food and Drug Administration (FDA) Dental Products Advisory Panel. Comments printed in this article do not reflect the policies of the FDA. Dr. Suzuki received no honorarium from Millennium Dental Technologies for this article.
About the Author
Jon B. Suzuki, DDS, PhD, MBA Professor of Microbiology and Immunology, School of Medicine, and Professor of Periodontology and Oral Implantology, School of Dentistry, Temple University, Philadelphia, Pennsylvania; Chairman and Director of Graduate Periodontology and Oral Implantology, Temple University; Associate Dean for Graduate Education, Temple University
References
1. White GS. Treatment of the edentulous patient. Oral Maxillofac Surg Clin North Am. 2015;27(2):265-272.
2. Compagnoni MA, Paleari AG, Rodriguez LS, et al. Impact of replacing conventional complete dentures with implant-supported fixed complete dentures. Int J Periodontics Restorative Dent. 2014;34(6):833-839.
3. Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17(1):5-15.
4. Chee WW. Treatment planning: implant-supported partial overdentures. J Calif Dent Assoc. 2005;33(4):313-316.
5. Doundoulakis JH, Eckert SE, Lindquist CC, Jeffcoat MK. The implant-supported overdenture as an alternative to the complete mandibular denture. J Am Dent Assoc. 2003;134(11):1455-1458.
6. Dierens M, Vandeweghe S, Kisch J, et al. Long-term follow-up of turned single implants placed in periodontally healthy patients after 16-22 years: radiographic and peri-implant outcome. Clin Oral Implants Res. 2012;23(2):197-204.
7. Gatti C, Gatti F, Chiapasco M, Esposito M. Outcome of dental implants in partially edentulous patients with and without a history of periodontitis: a 5-year interim analysis of a cohort study. Eur J Oral Implantol. 2008;1(1):45-51.
8. Daubert DM, Weinstein BF, Bordin S, et al. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86(3):337-347.
9. American Academy of Periodontology Task Force on Peri-Implantitis. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84(4):436-443.
10. Nicholson K, Blodgett K, Braga C, et al. Pulsed Nd:YAG laser treatment for failing dental implants due to peri-implantitis. In: Rechmann P, Fried D, eds. Lasers in Dentistry XX. Bellingham, WA: SPIE – The International Society for Optical Engineering; 2014:89290H-1–89290H-14.
11. Gold SI, Vilardi MA. Pulsed laser beam effects on gingiva. J Clin Periodontol. 1994;21(6):391-396.
12. Ting CC, Fukuda M, Watanabe T, et al. Morphological alterations of periodontal pocket epithelium following Nd:YAG laser irradiation. Photomed Laser Surg. 2014;34(12):649-657.
13. Harris DM, Yessik M. Therapeutic ratio quantifies antisepsis: ablation of Porphyromonas gingivalis with dental lasers. Lasers Surg Med. 2004;35(3):206-213.
14. Cobb CM, McCawley TK, Killoy WJ. A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol. 1992;63(8):701-707.
15. McCawley TK, McCawley MN, Rams TE. LANAP immediate effects in vivo on human chronic periodontitis microbiota. J Dent Res. 2014;93(spec iss A):Abstract 428.
16. de Andrade AKP, Feist IS, Pannuti CM, et al. Nd:YAG laser clinical assisted in class II furcation treatment. Lasers Med Sci. 2008;23(4):341-347.
17. Giannelli M, Bani D, Viti C, et al. Comparative evaluation of the effects of different photoablative laser irradiation protocols on the gingiva of periodontopathic patients. Photomed Laser Surg. 2012;30(4):222-230.
18. Giannini R, Vassalli M, Chellini F, et al. Neodymium:yttrium aluminum garnet laser irradiation with low pulse energy: a potential tool for the treatment of peri-implant disease. Clin Oral Implants Res. 2006;17(6):638-643.
19. Gonçalves F, Zanetti AL, Zanetti RV, et al. Effectiveness of 980-nm diode and 1064-nm extra-long-pulse neodymium-doped yttrium aluminum garnet lasers in implant disinfection. Photomed Laser Surg. 2010;28(2):273-280.
20. Kilinc E, Rothrock J, Migliorati E, et al. Potential surface alteration effects of laser-assisted periodontal surgery on existing dental restorations. Quintessence Int. 2012;43(5):387-395.
21. Stübinger S, Homann F, Etter C, et al. Effect of Er:YAG, CO2 and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg Med. 2008;40(3):223-228.
22. Geminiani A, Caton JG, Romanos GE. Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med Sci. 2012;27(2):339-342.
23. Geminiani A, Caton JG, Romanos GE. Temperature increase during CO2 and Er:YAG irradiation on implant surfaces. Implant Dent. 2011;20(5):379-382.
24. Gómez C, Domínguez A, García-Kass AI, García-Nuñez JA. Adjunctive Nd:YAG laser application in chronic periodontitis: clinical, immunological, and microbiological aspects. Lasers Med Sci. 2011;26(4):453-463.
25. Qadri T, Poddani P, Javed F, et al. A short-term evaluation of Nd:YAG laser as an adjunct to scaling and root planing in the treatment of periodontal inflammation. J Periodontol. 2010;81(8):1161-1166.
26. Giannelli M, Bani D, Tani A, et al. In vitro evaluation of the effects of low-intensity Nd:YAG laser irradiation on the inflammatory reaction elicited by bacterial lipopolysaccharide adherent to titanium dental implants. J Periodontol. 2009;80(6):977-984.
27. Arisu HD, Türköz E, Bala O. Effects of Nd:YAG laser irradiation on osteoblast cell cultures. Lasers Med Sci. 2006;21(3):175-180.
28. Chellini F, Sassoli C, Nosi D, et al. Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: “an in vitro study.” Lasers Surg Med. 2010;42(6):527-539.
29. Kim IS, Cho TH, Kim K, et al. High power-pulsed Nd:YAG laser as a new stimulus to induce BMP-2 expression in MC3T3-E1 osteoblasts. Lasers Surg Med. 2010;42(6):510-518.
30. Kim K, Kim IS, Cho TH, et al. High-intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J Tissue Eng Regen Med. 2015;9(8):943-951.