Amlodipine-Induced Gingival Enlargement—A Clinical Report
Abstract
Gingival enlargement is a known side effect of calcium channel blockers—especially the dihydropyridine group. It is a serious concern for both the patient and the clinician due to its unesthetic appearance and formation of new niches for periodontopathogenic bacteria. Among the calcium channel blockers, gingival enlargement has most frequently been described as a side effect following administration of nifedipine. The incidence with amlodipine is much lower; however, there have been few reports showing the association of this drug with gingival enlargement. This case report aims to make dentists aware of the adverse effects of amlodipine, as well offers a brief review of its effects on the gingiva and the management of enlargement.
“Gingival enlargement” or “gingival overgrowth” are the preferred terms for all medication-related gingival lesions previously termed “gingival hyperplasia” or “gingival hypertrophy.” These earlier terms did not accurately reflect the histologic composition of the pharmacologically modified gingiva. Gingival enlargement is a well-known consequence of administration of some anticonvulsants, the immunosuppressant drug cyclosporine A (CsA), and calcium channel blockers, and it may create speech, mastication, tooth eruption, and esthetic problems.1 Calcium channel blockers are used in the management of various cardiovascular disorders such as angina and hypertension. These drugs have been reported to be associated with gingival enlargement since 1984.2 Of this large group of drugs, the dihydropyridines—especially nifedipine—are most frequently implicated in gingival enlargement. The prevalence with amlodipine, 33.3%, is much lower.3 Seymour et al4 were the first to report on amlodipine-induced gingival overgrowth, and there have been only few reported associations of gingival overgrowth with this drug.
Case Report
A 55-year-old female patient reported to the Department of Periodontics at Subharti Dental College and Hospital, Meerut, India, with a chief complaint of swelling in the gums, which had been progressively increasing over the previous 6 months. The patient’s medical history revealed that she had been hypertensive for 6 years and was receiving a single dose of amlodipine 5 mg/day orally for the past 2 years.
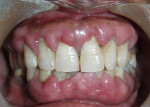
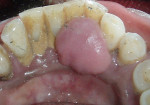
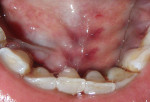
Intraoral examination revealed generalized pink gingiva with rolled gingival margins, lobulated papillae, and fibrous overgrowth throughout the maxilla and mandible, particularly on the labial and buccal side (Figure 1). A prominent nodular growth was also seen on the lingual aspect of the mandibular left incisors, which was approximately 1.5 cm x 1.5 cm in size, firm on palpation, and had a sessile base (Figure 2). Generalized deep pockets, exudation on application of digital pressure, and bleeding on probing was noted. The oral hygiene status of the patient was poor, accompanied by marked plaque and calculus accumulation around all teeth. Clinically, the differential diagnosis for the localized growth included pyogenic granuloma, fibroma, and peripheral ossifying fibroma. A provisional diagnosis of drug-induced gingival enlargement was made for the patient.
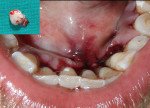
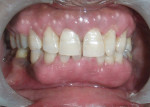
The patient was advised to substitute or discontinue the medication in consultation with her physician. Oral prophylaxis was done, and plaque control was emphasized to the patient. Two weeks later, the growth was excised from the lingual aspect of mandibular incisors and sent for histopathologic examination (Figure 3), after which the area was sutured with 3-0 silk. The patient was recalled after 1 week for suture removal. At the 3-month recall, the patient showed significant resolution of gingival inflammation on the buccal aspect (Figure 4) and uneventful healing on the lingual aspect (Figure 5).
Histologic examination of the specimen demonstrated hyperkeratinized and proliferating stratified squamous epithelium. The underlying connective tissue showed bundles of collagen fibers with an admixture of mild chronic inflammatory infiltrate and a small number of blood vessels. In the deeper connective tissue, severe chronic inflammatory infiltrate with some nerve bundles and blood vessels were seen (Figure 6). Histologically, the lesion was diagnosed as fibroepithelial hyperplasia, and was indicative of amlodipine-induced gingival hyperplasia based on clinical and histological evidence.
Discussion
Gingival enlargement, along with its cosmetic implications, also provides a niche for growth of microorganisms and is a serious concern for both the patient and clinician. Drugs associated with gingival overgrowth can be categorized broadly into three major groups according to their therapeutic actions, namely: anticonvulsants, for control of epileptic convulsions and other neurologic disorders; immunosuppressants, used for prevention of transplant rejection as well as for management of a number of autoimmune conditions such as rheumatoid arthritis; and calcium channel blockers, to ameliorate the nephrotoxicity of immunosuppressive agents or as part of a hypertensive regimen. Drugs associated with gingival enlargement, their brand names, and estimated prevalence rates are summarized in Table 1.5-15
Calcium channel blockers are considered potential etiologic agents for drug-induced gingival enlargement. Amlodipine is a third-generation dihydropyridine calcium antagonist, which has a mode of action that is pharmacodynamically comparable to nifedipine. However, amlodipine has a unique physiochemical profile, which is characterized by nearly complete resorption, late peak plasma concentrations, high bioavailability, and slow hepatic degradation. The associated slow elimination of amlodipine, with resulting long duration of its action, has resulted in better patient compliance, and has until now been associated with similar or reduced severity of side effects compared to nifedipine.3 Amlodipine, like nifedipine, can be detected in gingival crevicular fluid, and gingival sequestration of amlodipine associated with gingival hyperplasia has also been reported.4
Clinically, drug-induced gingival overgrowth normally begins at the interdental papillae and is more frequently found in the anterior segment of the labial surfaces.16 Gradually, gingival lobulations are formed that may appear inflamed or more fibrotic in nature, depending on the degree of local-factor-induced inflammation. The fibrotic enlargement normally is confined to the attached gingiva, but it may extend coronally and interfere with esthetics, mastication, or speech.17 Most drug-associated gingival enlargements appear to be clinically indistinguishable, with the possible exceptions of CsA8 and phenobarbitone.9 In phenobarbitone-treated patients, the gingiva may be enlarged uniformly without lobulations of the interdental papillae, and severity of the clinical lesions has been reported to be greater in the posterior as compared to the anterior regions.9 In individuals immunosuppressed with CsA, sometimes pebbly or papillary lesions appear on the surface of larger lobulations,18 which have been associated with the presence of candida hyphae invading the gingival epithelium.18 Other investigators have reported that tissues affected by CsA are generally more hyperemic and bleed more readily upon probing than tissues affected by phenytoin.8 The case described here is unique in that it presents with a solitary nodular growth on the lingual aspect of the mandibular incisors, along with generalized gingival enlargement on the facial/buccal aspect. Triveni et al19 reported a similar case of drug-induced gingival enlargement.
The histopathology of the lesions in all these drug categories is similar and is characterized by excessive accumulation of extracellular matrix proteins, such as collagen, or amorphous ground substance.7,16,17 Histologically, drug-induced gingival enlargement consists of pronounced hyperplasia of the connective tissue and epithelium. There is acanthosis of the epithelium, and elongated rete pegs extend deep into the connective tissue, which exhibits densely arranged collagen bundles with an increased number of fibroblasts and new blood vessels. Varying degrees of inflammatory infiltrate exist, with plasma cell as the predominant cell type.1 The mature phenytoin enlargement has a fibroblast/collagen ratio equal to that of normal gingiva from normal individuals, suggesting that at some point in development of the lesion, fibroblastic proliferation must have been abnormally high. Oxytalan fibers are numerous beneath the epithelium and in areas of inflammation. Cyclosporine enlargement usually have a more highly vascularized connective tissue with foci of chronic inflammatory cells, particularly plasma cells,20 plus presence of an abundant amorphous extracellular substance.1 Nifedipine-induced gingival hyperplasia also resembles phenytoin-induced gingival overgrowth histochemically. Gingival fibroblasts from both conditions contain strongly sulphated mucopolysachharides and numerous secretory granules.20
The pathogenesis of gingival overgrowth is uncertain. It has been proposed that susceptibility or resistance to pharmacologically induced gingival enlargement may be governed by the existence of differential proportions of fibroblast subsets in each individual who exhibits a fibrogenic response to these medications.21 In support of this hypothesis, it has been shown that functional heterogeneity exists in gingival fibroblasts in response to various stimuli.21 That not all patients taking calcium channel blockers develop overgrowth suggests a genetic predisposition.5 It is also believed that nifedipine and other chemically related drugs share the capacity to alter calcium metabolism at the cellular level.22 The influx of calcium ions across the cell membrane is thought to decrease due to reduced membrane permeability; and with the decreased influx of calcium, the secretory function of affected fibroblastic cells or collagenases production is reduced or inhibited. Thus, increased fibroblastic proliferation and collagen synthesis occurs.23
Inflammatory and non-inflammatory mechanisms have also been suggested. The non-inflammatory mechanisms include defective collagenase activity due to decreased uptake of folic acid, up-regulation of keratinocyte growth factor, blockade of aldosterone synthesis in the adrenal cortex, and consequent feedback increase in adrenocorticotropic hormone (ACTH) levels. Inflammatory mechanisms include direct toxic effects of the concentrated drug in gingival crevicular fluid and/or bacterial plaques. This inflammation could lead to up-regulation of various cytokines such as TGF-β1.24 Also, Seymour stated that the interaction between the drug and gingival tissues appears to be enhanced by gingival inflammation caused by inadequate oral hygiene.25
Treatment
The clinician should emphasize plaque control as the first step in the treatment of drug-induced gingival enlargement, as it may regress with time in patients undergoing a vigorous oral hygiene program.26 Usually, a 3-month interval for periodontal maintenance therapy has been recommended for patients taking drugs associated with gingival enlargement.27 Further consideration should be given to the possibility of medication withdrawal or substitution in patients for whom the new medication can offer some advantage for control of their hypertension, or in patients who present with clinically significant overgrowth and are at high risk of corrective surgery or recurrence after gingivectomy. Reduction of gingival overgrowth has been reported where substitution is made by a structurally different antihypertensive drug, including the angiotensin-converting enzyme inhibitor, enalapril; the β-blocking drug, atenolol; or thiazide diuretics.28 With this form of treatment, it may take from 1 to 8 weeks for resolution of gingival lesions.18
The primary aim of nonsurgical approaches such as scaling and root planing is to reduce the inflammatory component in the gingival tissues and thereby avoid the need for surgery.29 Surgical approaches such as gingivectomy are commonly performed for esthetic reasons before any functional consequences are present or when overgrowth is severe. Flap surgery/internal bevel gingivectomy appears to be more suited for cases with mild to moderate overgrowth associated with bone and attachment loss.17 The use of carbon dioxide lasers has shown some utility for reducing gingival enlargement, an approach that provides rapid postoperative hemostasis.7
In the presented case, amlodipine 5 mg/day was replaced with atenolol 50 mg/day twice daily by the physician. Based on the clinical evaluation of the soft-tissue response to scaling and the persistence of fibrotic component on the lingual aspect of mandibular incisors, gingivectomy was performed to remove the localized growth from the lingual aspect of mandibular incisors. The patient was placed on periodic recall of 3 months for the evaluation of the gingival condition and showed significant resolution of gingival inflammation on both buccal and lingual aspects, and, hence, the need for surgery was limited.
Conclusion
The reported case is an example of a combined type of gingival enlargement—basically a drug-induced one, complicated by inflammatory changes due to plaque accumulation. It seems likely that the use of medications for treatment of medical conditions and the potential to cause gingival overgrowth will increase in the years to come. Dentists should have a clear understanding of the drugs that cause this phenomenon and know how to manage these cases. If possible, treatment should focus on drug substitution and effective control of local inflammatory factors such as plaque and calculus. When these measures fail to cause resolution of the enlargement, surgical intervention is recommended. These treatment modalities, although effective, do not necessarily prevent recurrence of the lesions.
References
1. Carranza FA, Hogan EL. Gingival enlargement. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza’s Clinical Periodontology. 10th ed. St. Louis, MO: Saunders; 2006: 375-376.
2. Lederman D, Lumerman H, Reuben S, Freedman PD. Gingival hyperplasia associated with nifedipine therapy. Report of a case. Oral Surg Oral Med Oral Pathol. 1984;57(6):620-622.
3. Jorgensen MG. Prevalence of amlodipine-related gingival hyperplasia. J Periodontol. 1997;68(7):676-678.
4. Seymour RA, Ellis JS, Thompson JM, e al. Amlodipine induced gingival overgrowth. J Clin Periodontol. 1994;21(4):281-283.
5. Seymour RA, Thompson JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23(3 Pt 1):165-175.
6. Angelopoulos AP, Goaz PW. Incidence of diphenylhydantoin gingival hyperplasia. Oral Surg Oral Med Oral Pathol. 1972;34(6):898-906.
7. Marshall RI, Bartold PM. A clinical review of drug-induced gingival overgrowths. Aust Dent J. 1999;44(4):219-232.
8. Seymour RA, Heasman PA. Drugs, Diseases and the Periodontium. Oxford: Oxford University Press; 1992.
9. Gregoriou AP, Schneider PE, Shaw PR. Phenobarbital-induced gingival overgrowth? Report of two cases and complications in management. ASDC J Dent Child. 1996;63(6):408-413.
10. Katz J, Givol N, Chaushu G, et al. Vigabatrin-induced gingival overgrowth. J Clin Periodontol. 1997;24(3):180-182.
11. Romito GA, Pustiglioni FE, Saraiva L, et al. Relationship of subgingival and salivary microbiota to gingival overgrowth in heart transplant patients following cyclosporine A therapy. J. Periodontol. 2004;75(7):918-924.
12. Barak S, Engelberg IS, Hiss J. Gingival hyperplasia caused by nifedipine: Histolopathologic findings. J Periodontol. 1987;58(9):639-642.
13. Ellis JS, Seymour RA, Steele JG, et al. Prevalence of gingival overgrowth induced by calcium channel blockers: a community-based study. J Periodontol. 1999;70(1):63-67.
14. Andriani F, Margulis A, Lin N, et al. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Invest Dermatol. 2003;120(6):923-931.
15. Meller AT, Rumjanek VM, Sansone C, Allodi S. Oral mucosa alterations induced by cyclosporine in mice: morphological features. J Periodontal Res. 2002;37(6):412-415.
16. Hallmon WW, Rossmann JA. The role of drugs in the pathogenesis of gingival overgrowth. A collective review of current concepts. Periodontol 2000. 1999;21:176-196.
17. Dongari A, McDonnell HT, Langlais RP. A clinical review of drug-induced gingival overgrowth. Oral Surg Oral Med Oral Pathol. 1993;76(4):543-548.
18. Khocth A, Schneider LC. Periodontal management of gingival overgrowth in the heart transplant patient: a case report. J Periodontol. 1997;68(11):1140-1146.
19. Triveni MG, Rudrakshi C, Mehta DS. Amlodipine induced gingival overgrowth. J Ind Soc Periodontol. 2009;13(3):160-163.
20. Lucas RM, Howell IP, Wall BA. Nifedipine-induced gingival hyperplasia: A histochemical and ultrastructural study. J Periodontol. 1985;56(4):211-215.
21. Hassel TM, Page RC, Narayanan AS, Cooper CG. Diphenylhydantoin (dilantin) gingival hyperplasia: drug-induced abnormality of connective tissue. Proc Natl Acad Sci USA. 1976;73(8):2909-2912.
22. Ramon Y, Behar S, Kishon Y, Engelberg IS. Gingival hyperplasia caused by nifedipine—a preliminary report. Int J Cardiol. 1984;5(2):195-206.
23. Nery EB, Edson RG, Lee KK, et al. Prevalence of nifedipine-induced gingival hyperplasia. J Periodontol. 1995;66(7):572-578.
24. Lafzi A, Farahani RM, Shoja MA. Amlodipine-induced gingival hyperplasia. Med Oral Patol Oral Cir Bucal. 2006;11(6):E480-482.
25. Seymour RA. Calcium channel blockers and gingival overgrowth. Br Dent J. 1991;170(10):376-379.
26. Montebugnoli L, Servidio D, Bernardi F. Role of time in reducing gingival overgrowth in heart transplanted patients following cyclosporine therapy. J. Clin Periodontol. 2000;27(8):611-614.
27. Hall EE. Prevention and treatment consideration in patients with drug-induced gingival enlargement. Curr Opin Periodontol. 1997;4:59-63.
28. Mavrogiannis M, Ellis JS, Thomason JM, Seymour RA. The management of drug induced gingival overgrowth. J Clin Periodontol. 2006;33(6):434-439.
29. Somacarrera ML, Lucas M, Scully C, Barrios C. Effectiveness of periodontal treatments on cyclosporine induced gingival overgrowth in transplant patients. Br Dent J. 1997;183(3):89-94.
About the Authors
Shivani Sharma, MDS
Senior Lecturer
Department of Periodontics
Kanti Devi Dental College
Mathura, Uttar Pradesh, India
Anamika Sharma, MDS
Professor
Head of Department
Department of Periodontics
Subharti Dental College
Meerut, Uttar Pradesh, India