A Review of Dental Tissue Microstructure, Biomodification, and Adhesion
Abstract
Advancements in biomaterials and adhesive technology, along with the progression toward conservative, less invasive dental procedures, require a thorough knowledge of the micromorphology of dental tissues. This article provides a description of the morphological, histological, and physiological characteristics of the dental tissue substrates, explains the interplay between biomodification of the tissue and adhesion, and reviews the adhesion strategies at the restorative interface and criteria for its improvement.
Complete understanding of the unique characteristics of enamel and dentin tissues is essential for the optimal adhesion of the different restorative materials to these surfaces. It is also necessary as the basis for more conservative preparation designs and to preserve more tooth structure while increasing the longevity of adhesive restorations.
Characteristics of Enamel
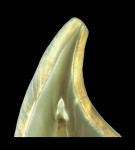
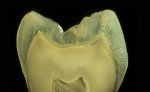
The anatomic crown of a tooth is comprised of an acellular calcified material known as enamel, which is the hardest tissue in the body.1 The thickness of the enamel varies according to the shape of the tooth and its location on the crown. The thickest area of enamel is normally located at the crest of the cusp or incisal edges, whereas the thinner regions are usually over the slope, at the cervix, or within the fissures and pits of multi-cuspid teeth1 (Figure 1A and Figure 1B). Human adult enamel, an inert, high-energy crystalline structure with high intermolecular forces, has been called a composite bioceramic.2 Enamel is a highly brittle and rigid structure because it possesses a high modulus of elasticity and low tensile strength.3 Dental enamel is acellular and does not regenerate.1
The largest basic structural component is the enamel prism or rod, densely packed and intertwined in a wavy course extending from the dentoenamel junction (DEJ) up to a few micrometers short of the enamel tooth surface.3 The ideal structure of these prisms is a keyhole-like configuration with an average width of about 0.5 µm. The rods appear in a transverse section as a rounded head or body section and as a tail section forming a repetitive series of interlocking prisms. The enamel prism is narrowest at its origin and gradually enlarges as it approaches the surface, with an average diameter of 4.0 µm. Enamel prisms are arranged parallel to each other and run outward from the DEJ in a radial pattern, approximately perpendicular to the external surface of the crown. In the region of the enamel cusps, the rod orientation is perpendicular to the DEJ, while in the cervical region (ie, where the contours of the crown become constricted), prisms exhibit a gingival or apical inclination.3 In the incisal or occlusal third, the prisms form an increasingly acute angle with the surface as the tip of the crown is approached, exhibiting an orientation that is more directly opposed to the forces of mastication.1,4 This basic knowledge is important during cavity preparation in order to avoid undermining enamel rods, which can fracture along cleavage planes paralleling the course of the enamel prisms. It also supports the clinician's decision to incorporate bevels in adhesive preparation designs in order to provide union at the ends of the enamel rods instead of at their long axis, which increases surface area and provides strength and greater retention.5
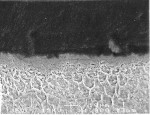
The enamel prisms are filled with millions of small, elongated, tightly packed carbonated apatite crystallites that vary in size and shape.3,6 The enamel apatite, hexagonal in cross-section, is the largest and longest biological crystal4 (Figure 2). These enamel crystallites are tightly packed in a distinct orientation pattern that provides structural identity and strength to the prisms. The long axis of the apatite crystallites within the central region of the head is aligned almost parallel to the rod long axis, but in the tail region, the crystallites incline with increasing angles (ie, as much as 65°) to the prism axis. The susceptibility of these crystallites to acid (eg, from caries or etching procedures) appears to be related to their orientation,3 resulting in differential etching patterns that facilitate micromechanical retention. The interfacial area is a protein-rich section between the prisms or rods, which is termed interprismatic substance or inter-rod enamel.3,6-8 Encompassing each enamel rod is an organic matrix or prism sheath, which is an organically rich interspace.3,6 This sheath may be an increased spacing between differently oriented crystallites so that the "tail" portion of one rod meets the "head" portion of another.1,3,8
Characteristics of Dentin
The dentin is a living tissue and constitutes the largest portion of tooth structure. Its microstructure has been characterized by scanning and transmission electron microscopy, atomic force microscopy, visible microscopy, and various methods of surface analysis.9,10 The past two decades of research have expanded knowledge of the nature of dentin and the dentin-restorative material interface.11 Dentin's microstructure is substantially different from that of enamel. Dentin is similar to bone and cementum in that it originates from mesenchyme cells known as odontoblasts. The dentin is a specialized calcified connective tissue that is harder and denser than bone.1,3,6,8 Its surfaces are bordered externally by enamel and cementum, and internally by the odontoblastic layer. The dentin has been considered part of the pulp-dentin complex by many researchers and the odontoblast as a part of both, with the mineralized dentin an end product of cell differentiation and maturation.3
While dentin and enamel have similar inorganic crystallite constituents, there is a considerable difference in their organic component and percentage of composition.12 The dentin has been referred to as a "composite" of hydroxyapatite, collagen, and water.13 Volumetrically, about 50% of the dentin is composed of hydrated organic material.14 The inorganic portion of the dentin is composed of hydroxyapatite crystallites arranged with less uniformity than enamel crystallites. Similar in size to those of bone and cementum, the dentinal crystallites are smaller, thinner, and needle-shaped.1,3,15 A large percentage of these crystals are interspersed among the collagen fiber network to reinforce the collagen matrix.4 The individual fibrils within the collagen fibers also have crystals at their ends. The lower mineral composition gives the dentin a low modulus of elasticity (ie, 1.67 x 106 PSI), which allows more elastic deformation during loading of the more brittle, nonresilient enamel.3,4
The structural and morphological entities of the dentin include the dentin tubule, the peritubular dentin, the intertubular dentin, the odontoblast with its odontoblastic process, and the periodontoblastic space (Figure 3).15 The structure of dentin consists of a calcified matrix penetrated by tubules that extend from the pulp to the DEJ and are formed at a slight angle to the DEJ and pulp chamber.4 The direction of the tubules are sigmoidally curved and follow a gentle "S" curve in the coronal regions of the crown; their path is straighter in direction in the incisal ridges, cusps, and root areas.1,8 The average diameter of the dentinal tubules varies in size from 0.5 µm to 0.9 µm at the DEJ and from 2.0 µm to 3.0 µm at the pulp interface.4 The concentration of tubules is higher along the inner or deep dentin (ie, approximately 45,000 to 65,000 tubules/mm2) than at the middle dentin (ie, approximately 35,000 tubules/ mm2), or the superficial dentin (ie, approximately 15,000 to 20,000 tubules/ mm2).4 Therefore, the surface area of dentin is larger at the dentinoenamel or dentinocemental junction than at the pulp. The entire length of the dentinal tubules is lined by a sheet-like structure called the lamina limitans,15 and each tubule contains the cytoplasmic cell process (ie, Tomes' fiber) of an odontoblast. These cytoplasmic extensions extend at least one third into the dentin, and may extend the entire length of the tubules, which is why the dentin has been regarded as an anatomical and physiological extension of the pulp12 (Figure 4). Additionally, these tubules are filled with a tissue fluid, a transudate of plasma referred to as the dentinal fluid or dentin liquor.3,15 The primary dentin tubules are often interconnected by an intercommunicating and anastomosing network of laterally secondary tubules and are generally more numerous in the dentin of the root than in the coronal dentin.3,15,16
The matrices in dentin include the predentin, which is a nonmineralized zone of developing dentin adjacent to the odontoblasts, and the mineralized mature dentin. This nonmineralized matrix surrounds the odontoblastic process and is an essential exchange medium; in combination with the odontoblasts process, it forms the soft components of dentin called the periodontoblast matrix. In the dentinal tubules, the nonmineralized matrix separates the protoplasmic processes from the tubule wall.1 The calcified mature dentin has three different mineral densities: low, moderate, and high. Characterized by its high mineral content, the high-density dentin (ie, the peritubular dentin) encircles the tubule and forms the tubule wall. The high-mineralized peritubular dentin varies in width depending on several factors (eg, the age of the tooth), and may completely obliterate the tubules. The intertubular dentin is found between the peritubular dentin of adjacent tubules and has a matrix of moderate mineral density and abundant collagen15 (Figure 4). The zone of low mineral content is at the calcifying front between the predentin and the dentin. The collagen fibers are mainly found in the intertubular dentin; only a sparse amount of organic material is present in the peritubular and periodontoblastic space.15
Unlike enamel, which is unable to develop once the enamel rod forms and the ameloblast degenerates, dentin continues to form after tooth eruption and development and throughout the life of the pulp. As long as the odontoblastic cell processes are present, the formation of dentin (ie, dentinogenesis) will continue at different rates, depending upon the biological and functional demand.1 These cell processes are the reason dentin is considered a living tissue with the capability to react to physiologic and pathologic stimuli. These stimuli can result in changing structural features and dentin thickness throughout the life of the tooth, with the formation of secondary dentin, reparative dentin, sclerotic dentin, and dead tracts.1, 3
Biomodification and Adhesion to Dental Tissue Substrates
The biomodification (ie, chemical treatment) of enamel and dentin by buffered acids facilitates interdiffussion of resins into the dental tissue substrates and has been the standard clinical procedure in adhesive dentistry since the 1960s. Acids can remove the smear layer either completely or partially. This raises the surface energy of the tissue, alters the mineral content of the substrates, and the created microretentions can be subsequently infiltrated by primers and bonding resins. The mechanism of adhesion is similar for enamel and dentin (ie, a micromechanical entanglement of monomers into the enamel microporosities or collagen interfibrilar spaces occurs) (Figure 5A and Figure 5B).
Enamel
Acid etching of enamel has become a standard procedure for resin-bonded interfaces because of its demonstrated ability to obtain an effective micromechanical bond of 20 MPa or more between resin and tooth enamel17 (Figure 6A and Figure 6B). Note: 19 MPa is considered the minimal clinically acceptable bond strength. While various studies have been undertaken to improve the depth of the etched zone or the amount of surface enamel removed during etching, a 15-second etch with 37% phosphoric acid is generally sufficient to produce microtags that facilitate the bonding process.17 When evaluating restorative success, the marginal integrity achieved from this enamel acid-etching procedure becomes a priority, since an intact restorative tooth interface is essential to the exclusion of bacteria and the interfacial hydrodynamic equilibrium of the dentinopulpal complex.
Dentin
Contemporary bonding philosophies adopt two different adhesive strategies (ie, total-etch and self-etch) for successful bonding to dentin.18 Both require an understanding of the altered morphology of the dentin and its composition after instrumentation (ie, smear layer), but differ in the method in which it is treated and the adhesive system utilized. A better understanding of the smear layer is necessary for a complete evaluation of the two strategies and their respective adhesive systems.
The smear layer appears on the dentin surface after instrumentation and consists of "altered morphology" and "various particulate debris."19 The debris is burnished against and bound to the surfaces of the dentin during instrumentation for cavity preparation. The altered morphology of the dentin includes a roughened, smeared appearance with obliteration of the tubule orifices. This may be a result of heat generation and plastic and elastic deformation of the surface during the cutting procedure.19, 20 The particulate debris is composed of saliva, blood, and micro-organisms, as well as ground enamel and dentin20 (Figure 7). The thickness of the smear layer (ie, approximately 0.5 µm to 5.0 µm) depends on the type of cutting instrument used.20 Depending on how it is generated, the surface topography may vary. Diamond burs produce a rougher surface than carbide burs, and dry cutting produces greater smearing and roughness than wet cutting21,22 (Figure 8A and Figure 8B). The smear layer occludes the dentinal tubules and acts as a "diffusion barrier" that decreases dentinal permeability; it can be considered an obstruction that prevents resin from reaching the underlying substrate.23
Total-etch Technique
The total-etch technique requires the application of an acid or calcium chelators that decalcify the outer layer of dentin and a separate application of a primer and adhesive. The acid-etching process removes the smear layer and the dentinal tubule plugs, increases dentinal permeability, and decalcifies the intertubular and peritubular dentin (Figure 9). The removal of the inorganic support (ie, the hydroxyapatite crystals) leaves a network of collagenous fibrils exposed, which collapses on the deep, undecalcified inorganic dentin support.24,25
After the conditioner is washed off, a primer containing one or more hydrophilic monomers is applied. These primer molecules [ie, hydroxy-ethyl-methacrylate (HEMA), biphenyl dimethacrylate (BPDM), and 4-methacryloxyethyl trimellitate anhydride (4-META)] contain two functional groups: a hydrophilic and hy-drophobic. The hydrophilic group has an affinity for the water content within the dentin; the hydrophobic group has an affinity for the resin monomer. The primer wets and penetrates the collagen network. An unfilled adhesive resin is applied to and penetrates into the primed dentin, copolymerizing with the primer to form an interlock with the dentinal matrix. The primary bonding mechanism of many current adhesive systems, this layer of polymerized resin intermingled with collagen fibers has been demonstrated and previously defined as the resin-reinforced dentin, resin-reinforced zone, resin-infiltrated layer, or hybrid layer.24,25
Self-etch Technique
The self-etching primer/adhesive strategy leaves the smear layer in place. These dentin bonding systems are based on the infiltration and modification of the smear layer by a weaker acidic monomer compared to the stronger total-etch counterpart that completely dissolves it. The objective is to incorporate the smear layer into the hybridized demineralized dentin. These less acidic, higher pH hydrophilic primers penetrate the smear layer and achieve micromechanical bonding of the infiltrating adhesive monomers to the underlying demineralized dentin. This technique allows the simultaneous infiltration of the collagen fibers and decalcified tissue to the same depth in dentin, thus minimizing the risk of not reinforcing part of the demineralized dentin (Figure 10). Additionally, this prevents the collapse of the collagen fibrils after conditioning and drying. The resin may slightly infiltrate (ie, 0.1 µm to 0.5 µm) the smear layer and the dentin and copolymerize.26
The clinical advantages reported with the self-etch compared to the total-etch adhesive technique include a simplified protocol and reduced postoperative sensitivity. However, there is only limited clinical data on the longevity of the bonds. Self-etch systems do not require a separate acid-etching step and the washing off of acid.
Self-etch adhesives are less technique sensitive than the acid-etch adhesives in the categories that follow:
- The technique sensitivity associated with substrate hydration is eliminated, since water is a fundamental ingredient of these self-etch systems, allowing ionization of the acidic monomers for demineralization of hard dental tissues.27
- In comparison to acid-etch adhesives, self-etch adhesives do not allow a discrepancy between the depth of demineralization and the depth of resin infiltration because both processes occur simultaneously.28
- Since the smear plugs are not removed before the application of the adhesive, the potential for postoperative sensitivity is less than with total-etch adhesives.28
- Finally, a dentin primer is required on enamel with multi-bottle total-etch adhesives and the moist bonding technique. However, moist bonding is not required for self-etch adhesives. 28
Adhesion at the Restorative Interface
The word "adhesion" is derived from the Latin roots translating as "to" and "stick together." Defined as the "molecular attraction exerted between the surfaces of bodies in contact," the force referred to as adhesion occurs when unlike molecules are attracted.17 Conversely, cohesion occurs when molecules of the same kind are attracted. The adhesive, frequently a viscous fluid, is comprised of a material or film that joins together two substrates and solidifies them. The adherend is the material or initial substrate to which the adhesive is applied.17 In dentistry, a surface sealant would be defined as a single adhesive "joint," since only one interface exists. While most adhesive joints involve only two interfaces, a bonded composite restoration would be an example of a more complex adhesive joint.4 Ensuring adequate performance of the adhesive joint requires knowledge and experience in the types of adherends (ie, enamel, dentin, metal alloy, and composite material) and the nature of the surface pretreatment or primer. The adhesive, adherend, and surface all impact the durability of the bonded structure. The mechanical behavior of the bonded structure is influenced by the details of the joint design and by the way in which the applied loads are transferred from one adherend to the other. The specific energy of adhesion—defined by chemical, physical, and mechanical attributes of the substrate and adhesive—determines the ability to form a joint and the resistance of the joint to failure.4 Achievement of such interfacial molecular contact is a necessary first step in the formation of strong and stable adhesive joints. Inherent in the formation of an optimal adhesive bond is the ability of the adhesive to wet and spread on the adherends being joined. Good wetting usually occurs with solids that demonstrate high surface energy. Adhesives should exhibit low viscosities or low surface tension in order to increase their wetting capabilities.29
Once wetting is achieved, intrinsic adhesive forces are generated across the interface through mechanisms of mechanical interlocking, adsorption, diffusion, or any one of their combinations. Mechanical interlocking occurs when adhesive flows into pores in the adherend surface or around projections on the surface. In adsorption, adhesive molecules adsorb onto a solid surface and bond to it. This process may involve the chemical bonding between the resin (ie, adhesive) and the inorganic or organic elements (ie, adherend) of the tooth structure. Diffusion involves a mechanical or chemical bonding between polymer molecules (ie, resin) and a precipitation of substances on the tooth surface (ie, adherend). Most often, more than one of these mechanisms play a role in achieving the desired level of adhesion for various types of adhesive and adherend.4
The bonded restorative complex includes the outer layers of the substrate, the adhesive layer, and the restorative material. The latter, when properly joined to the tooth substrate, is able to provide an improved marginal seal while reducing marginal contraction gaps, microleakage, nanoleakage, marginal staining, and secondary caries.29 The adhesion between tooth and biomaterial also results in restoration retention and a reduction of stress at the tooth-restorative interface. Biomechanically, this bond reinforces tooth structure and biologically preserves tissues, seals dentin tubules, and provides long-term functional success30-32 (Figure 11 and Figure 12).
The integrity of the hybridized zone is very important for the longevity of the bond. Any defect below or above the hybrid layer is critical. These separation zones typically occur below the hybrid layer as a consequence of excessive water remaining on the tissue, the "overwet phenomenon," or improper infiltration of the adhesive monomer. These defects at the bottom of the hybridized zone can eventually lead to nanoleakage and failure of the bond (Figure 13).
Criteria for Improving Adhesion at the Interface
Achieving success with adhesive restorations begins at the restorative interface. A durable interfacial adhesion between the tooth and biomaterial requires a clean surface of the substrate, a low contact angle that allows the adhesive to spread over the entire surface of the substrate, and optimal infiltration of adhesive monomers into the substrates.
The following treatment strategies are prerequisites for achieving a durable adhesive interface.
- Utilize dental dam isolation. Contamination of the enamel and dentin with saliva, moisture from intraoral humidity, blood, and crevicular fluid can compromise the longevity of the adhesive restoration by affecting the adhesion at the interface and reducing bond strengths.33
- imit the use of astringents or hemostatic agents, caries detectors, peroxides from bleaching agents, and zinc oxide and eugenol that can contaminate and reduce the bond strength by 50% or more.34
- Manage shrinkage stress at the interface by reducing cavity volume and intensity of curing lights, selecting low shrinkage composite resins, and utilizing stress-reducing restorative placement techniques.35
- Understand and develop knowledge of the kind of solvent (eg, water, ethanol, and acetone) in the adhesive and how it affects the application technique and the necessary moisture content needed in the dentin.
- Evaluate tooth composition during preparation (eg, ground vs. prepared enamel); dentin that has experienced microstructural modifications (ie, sclerotic dentin) presents challenges to consistent and predictable bonding.
- Preserve marginal integrity during finishing procedures. Finishing and polishing protocols can influence the longevity of adhesive restorations by affecting wear resistance.36 Reduced and/or delayed finishing may help to preserve the marginal integrity. Avoid aggressive cutting of tooth structure and mutilation of margins.
- Consider the application of a surface sealant to the interface. Application of a composite surface sealant after initial finishing may help to seal microcracks or microscopic porosities that may have formed during the procedure and has been shown to reduce the wear of posterior composite restorations.37
- Achieve occlusal stability prior to treatment. Interceptive occlusal equilibration and occlusal guard therapy prior to restorative treatment can reduce excessive interocclusal loading that can compromise the dimensional stability of the adhesive interface.
Conclusion
Since the initial mid-century discovery and introduction of biomodification of dental tissues by Hagger and Buonocore, restorative concepts have continued to evolve. The transition from a nonadhesive mentality of the G.V. Black era to the adhesive era resulted in a modification of the restorative mechanical paradigm to a biologic, minimally invasive approach that preserves tooth structure. The adhesive design concept requires an understanding of the biological microstructure of the dental tissues and influences the preparation design, restorative material selection, and material placement technique. The last half of the 20th century has evidenced two main bonding philosophies that implement biomodification as greatly influenced by the adhesive composition, tooth-surface mechanical treatment (ie, bur, laser, and hand instrument), and the micromorphology of the dental tissues. This evolution in surface biomodification has revolutionized the way clinicians practice restorative dentistry in their offices today.
Acknowledgements
The authors are grateful to Jorge Perdigão, DMD, MS, PhD, for his review of this article and the use of SEM images, and to Didier Dietschi, DMD, and Stephan J. Paul, DMD, for providing SEM images.
References
1. Provenza DV, Seibel W. Oral Histology: Inheritance and Development. 2nd ed. Philadelphia, PA: Lea and Febiger; 1986.
2. White SN, Luo W, Paine ML, et al. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 2001;80:321-326.
3. Roberson TM, Heymann HO, Swift EJ, et al. Sturdevant's Art and Science of Operative Dentistry. 4th ed. St. Louis, MO: Mosby; 2002.
4. Craig RG, Powers JM. Restorative Dental Materials. 11th ed. St. Louis, MO: Mosby; 2002.
5. Peters MC, McLean ME. Minimally invasive operative care. I. Minimal intervention and concepts for minimally invasive cavity preparations. J Adhes Dent. 2001;3:7-16.
6. Provenza DV. Oral Histology: Inheritance and Development. Philadelphia, PA: Lippincott; 1964.
7. Gilmore HW, Lund MR. Operative Dentistry. 2nd ed. St. Louis, MO: Mosby; 1973.
8. Schultz LC. Operative Dentistry. Philadelphia, PA: Lea and Febiger; 1966.
9. Boyde A, Lester KS. An electron microscope study of fractured dentinal surfaces. Calcif Tissue Res. 1967;1:122-136.
10. Williams RL, Williams DF. X-ray photoelectron spectroscopy (XPS) of dentin surfaces. J Dent Res. 1988;67: Abstract #269.
11. Butler WT. Dentin extracellular matrix and dentinogenesis. Oper Dent. 1992;Suppl 5:18-23.
12. Baratieri LN, Berry TG. Esthetics: Direct Adhesive Restoration on Fractured Anterior Teeth. Sao Paulo, Brazil: Quintessence; 1998.
13. Paul SJ. Adhesive Luting Procedures. Berlin, Germany: Quintessenz Verlags-GmbH; 1997.
14. Bowen RL, Marjenhoff WA. Development of an adhesive bonding system. Oper Dent. 1992;Suppl 5:75-80.
15. Söderholm KJ. Does resin based dentine bonding work? Int Dent J. 1995;45:371-381.
16. Mjör IA, Fejerskov O. Human Oral Embryology and Histology. 1st ed. Copenhagen: Munksgaard; 1986.
17. Anusavice KJ. Structure of matter and principles of adhesion. In: Phillips' Science of Dental Materials. 10th ed. Philadelphia, PA: W.B. Saunders Company; 1996.
18. Eick JD, Gwinnett AJ, Pashley DH, et al. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8: 306-335.
19. Eick JD, Wilko RA, Anderson CH, et al. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of the electron microprobe. J Dent Res. 1970;49 Suppl: 1359-1368.
20. Swift EJ Jr. A review of dentin bonding. Gen Dent. 1988;36: 396-399.
21. Gwinnett AJ. Smear layer: morphological considerations. Oper Dent Suppl. 1984;3:2-12.
22. Pashley DH. Smear layer: physiological considerations. Oper Dent Suppl. 1984;3:13-29.
23. Pashley DH, Michelich V, Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent. 1981;46: 531-537.
24. Van Meerbeek B, Inokoshi S, Braem M, et al. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J Dent Res. 1992;71:1530-1540.
25. Swift EJ Jr., Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. 1995;26: 95-110.
26. Eick JD, Robinson SJ, Chappell RP, et al. The dentinal surface: its influence on dentinal adhesion. Part III. Quintessence Int. 1993;24: 571-582.
27. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives. Part II: etching effects on unground enamel. Dent Mater. 2001;17: 430-444.
28. Perdigão J, Geraldeli S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J Esthet Restor Dent. 2003;15:32-42.
29. Armstrong SR, Boyer DB, Keller JC. Microtensile bond strength testing and failure analysis of two dentin adhesives. Dent Mater. 1998;14: 44-50.
30. Goracci G, Mori G. Esthetic and functional reproduction of occlusal morphology with composite resins. Compend Contin Educ Dent. 1999;20:643-648.
31. Van Meerbeek B, Vanherle G, Lambrechts P, et al. Dentin- and enamel-bonding agents. Curr Opin Dent. 1992;2:117-127.
32. Eakle WS. Fracture resistance of teeth restored with class II bonded composite resin. J Dent Res. 1986;65:149-153.
33. Powers JM, Finger WJ, Xie J. Bonding of composite resin to contaminated human enamel and dentin. J Prosthodont. 1995;4: 28-32.
34. Powers JM, O'Keefe KL, Pinzon LM. Factors affecting in vitro bond strength of bonding agents to human dentin. Odontology. 2003;91: 1-6.
35. Terry DA. Natural Aesthetics with Composite Resin. 1st ed. Mahwah, NJ: Montage Media Corporation; 2004.
36. Schwartz RS, Summitt JB, Robbins JW. Fundamentals of Operative Dentistry: A Contemporary Approach. Chicago, IL: Quintessence Pub.; 1996.
37. Dickinson GL, Leinfelder KF. Assessing the long-term effect of a surface penetrating sealant. J Am Dent Assoc. 1993;124:68-72.
a GC Cavity Conditioner, GC America Inc., Alsip, IL
b Keta™ Conditioner, 3M ESPE, St. Paul, MN
About the Authors
Douglas A. Terry, DDS
Assistant Professor, Department of Restorative Dentistry and Biomaterials
University of Texas Dental Branch
Houston, Texas
Private Practice
Houston, Texas
Cynthia P. Trajtenberg, DDS, MS
Assistant Professor, Department of Restorative Dentistry and Biomaterials
The University of Texas Dental Branch
Houston, Texas
Markus B. Blatz, DMD, Dr. Med Dent
Chairman, Department of Preventive and Restorative Sciences
Philadelphia, Pennsylvania
Professor of Restorative Dentistry
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
Karl F. Leinfelder, DDS, MS
Adjunct Professor, Department of Biomaterials Clinical Research
University of North Carolina–Chapel Hill
Chapel Hill, North Carolina
Professor Emeritus
University of Alabama School of Dentistry
Birmingham, Alabama