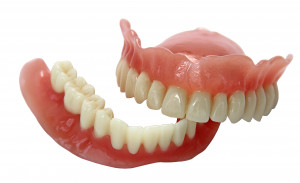
EnvisionTEC, a leading global manufacturer of desktop and full-production 3D printers and materials, today received FDA approval for its new E-Denture material for the 3D printing of lifelike dentures.
Combined with EnvisionTEC’s FDA-approved E-Dent 100 and 400 materials for the direct printing of restorations that simulate teeth, EnvisionTEC is now the only 3D printing company worldwide that offers a complete digital denture workflow solution. E-Dent is currently offered in four natural shades.
Dental labs and dentists can now combine a pink 3D printed denture base and tooth restoration into a denture that patients can wear for long-term use.“This new material expands EnvisionTEC’s already industry-leading dental materials library,” said CEO Al Siblani. “Even more importantly, E-Denture gives our 3D printer owners the ability to print even more materials.”
Both E-Denture and E-Dent can be 3D printed on EnvisionTEC’s Vida desktop and Perfactory production machines. Owners of those machines can already 3D print a variety of dental parts with EnvisionTEC materials:
E-Denture will be available for limited distribution in the third quarter.