A New Breed
Opportunities abound for the pioneering technologist with specialized education and knowledge.
It is uncertain how a shrinking dental laboratory industry will restructure to combat the pressures of being overtaken by big-store manufacturers, off-shore competition, and digital production. For many, the reality is that the choice will come down to re-educating and investing or slowly dying out. For those with the willingness and financial resources to confront the challenges, the evolutionary changes taking place in the dental industry are creating opportunities and giving birth to a new breed of dental technologist.
This new generation is not confined by the traditional definition that the dental industry has imposed upon it. These pioneers are transforming from conventional laboratory owners to specialists in select fields of the profession, in which technology and a superior knowledge base offer the opportunity to break away and redefine the role dental technology will play in the future of dentistry.
Among those who have ventured into this uncharted territory and advanced the field is Daniel Llop, CDT, president and chief executive officer of the nSequence Center for Advanced Dentistry (www.nsequence.com). Specializing in implant and esthetic dentistry, he has positioned his business for a digital future by carving out a unique business model for his implant-focused education and laboratory outsource center.
“We have married CBCT technology and CAD/CAM dentistry into a comprehensive treatment planning and fabrication process that offers clinicians and laboratories a complete digital implant resource from start to finish,” Llop said.
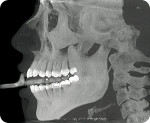
Patients are scanned in-house using the i-CAT® CBCT scanner (Imaging Sciences International Inc, www.imagingsciences.com). A full-time, certified dental X-ray technician and oral/maxillofacial radiologist takes and reads the scans and helps with the implant planning process. The center also accepts electronically transmitted DICOM data from remote CBCT scan centers to initiate the treatment-planning procedures.
They support all major digital implant-planning software systems. nSequence also has its own patented CT-guided planning and prosthesis design software protocol called nSoftt. This includes the Radiographic Registration Device (RRD), the virtual planning software, the AccuDental Validation Model, and the nSequence surgical guide. The nSoftt protocol bridges the gap seamlessly between DICOM and CAD/CAM by virtually creating all procedural and prosthetic fabrication from diagnostic waxup to custom patient-specific abutments, implant bars, and the final prosthesis as if it were created in the dental lab or chairside.
Llop and his research team have also developed advanced tools and techniques, specialized and patented for the surgical and restorative aspects of treatment, that promise increased predictability for the clinician and less surgery and a shorter recovery time for the patient. The facility manufactures various products, including a patented RRD custom-scan appliance to eliminate the need for custom-fabricated CBCT guides, implant system-specific nSequence surgical instrumentation kits, and 3-D printed AccuDental validation models for viewing key anatomical landmarks. They also offer the patent-pending Pisces patient-specific healing abutment, patent-pending Wave patient-specific zirconia metal-based abutment, and titanium-milled or electron-beam melted implant bars.
The nSequence Sequence
For laboratories that have dentists involved in digital implant planning but do not have the in-house capabilities to handle the digital aspects of the case, nSequence offers a flexible range of services to help laboratories get involved in the treatment planning process from the beginning—before the implants are placed. At any point, the laboratories can enter or exit the treatment planning, surgical guide production, provisionalization, and restorative phases of treatment. This gives them the freedom to choose which aspects of the case they wish to handle and which they want nSequence to complete.
All cases begin with the prescription and the digital data acquired for the case. “Each of our laboratory partners has a page on their lab’s website that is devoted to the nSequence implant-planning process that they use to initiate a case,” Llop explained. “They fill out the form with specifications for that particular case and submit it to us. In the meantime, the laboratory’s dentist uploads the patient’s CBCT DICOM data (Figure 1, Figure 2 and Figure 3), clinical digital photographs, panoramic X-rays, an optical impression capture (eg iTero), and other case-specific data to our website or sends us the physical impression and RRD tray.” The laboratory is notified that the case has arrived as soon as the dentist submits it to nSequence.
Utilizing Internet resources such as GoToMeeting online conferencing, the center brings operatory and laboratory specialists together to collaborate on a case (Figure 4, Figure 5, Figure 6 and Figure 7). If the laboratory has specific instructions on how the case should be handled, these instructions are relayed to the dentist so that all of the implant team members are involved in the process.
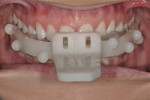
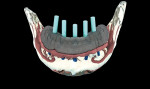
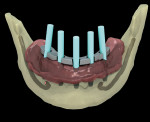
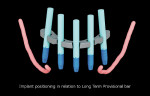
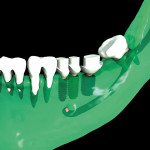
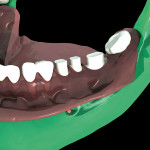
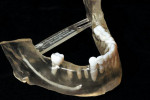
The surgical guide and patient-specific abutment may be created by the originating laboratory in-house, outsourced to a third party, or produced by nSequence using digital files submitted by the clinician (Figure 8). In the operatory, the dentist receives an AccuDental model (Figure 9) that is a physical representation of the CBCT scan combined with the optical impression scan data.
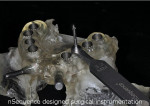
The model features critical anatomical landmarks such as the inferior alveolar nerve canal, tooth roots, bone abnormalities, and clear soft tissue to validate implant placement for the surgical team, which can improve the final case outcome for the patient. nSequence also provides custom-designed surgical instrumentation for implant placement (Figure 10). To help surgeons determine if the appropriate bone mass is present for implant placement, nSequence also has developed a 3-D printed, customized bone-reduction guide that can facilitate bone remodeling and implant placement with sub-millimeter accuracy.
On the Horizon
Because of its commitment to digital dentistry, the company is making a financial investment in the future by delving further into the arena of research and development. Currently, Llop is building an engineering wing onto the facility. “There is a new animal in the forest—it’s called the digital dental laboratory,” Llop said. “Here at nSequence, we employ an aerospace engineer, two biomedical engineers, two mechanical engineers, an IT specialist, a graphic artist, and three master dental technicians.”
Llop believes that investing in higher education is key to a company’s present and future growth. He and his staff continually seek learning opportunities in six of the seven major dental disciplines to keep up with the latest developments in the industry and stay ahead of the curve.
Down the road, Llop said the company will conduct research in areas such as how the application of patient-specific nano-materials to implant systems can aid in osseointegration. “Nano technology will play a huge role in dentistry. If they can make a car paint that heals itself when damaged, then it is only a short period of time before that technology is brought to other fields such as dentistry,” Llop said. “My prediction is that within 5 years, physical impressions will fade out and be replaced by optical impressions. And CBCT will be combined with other non-radiographic scanning technologies to capture both the hard and soft tissue in a single scan, rather than doing what we do now—combining the CBCT scan with the optical impression scan.”
He believes it is vital that dental technologists keep up with or get ahead of the industry to continue expanding their role as advisors to their client base. “At the end of the day, the dental laboratory is the liaison between the patient and the dentist,” Llop said. “That is something manufacturers and off-shore laboratories can never be.”