Trends in Nonsurgical Periodontal Treatment
Insights are offered into the techniques clinicians are using to treat periodontal diseases, and their evidence basis.
Dentists and dental hygienists have long relied on probing depths, clinical signs of inflammation (eg, bleeding on probing), and the extent and pattern of loss of clinical attachment and bone in their diagnosis of periodontal diseases.1 They have also offered patients a variety of periodontal services—most commonly, those that are nonsurgical in nature.2 As advances in the provision of oral healthcare continue to be achieved, new nonsurgical approaches in diagnostics, adjunctive modalities, and self-care techniques may help to arrest and even reverse the destruction of periodontal diseases without surgical intervention. Further understanding of the immunologic component of periodontal diseases offers guidance on when to initiate therapy and how to regulate the body’s immune response to oral infection.3 Additionally, greater understanding of the microbial etiology of periodontal diseases has led to anti-infective adjunctive therapies such as chemotherapeutic rinses, subgingival antimicrobial agents, and systemic antibiotic agents.4 Lasers have also become more common because of the possibility that they can remove bacteria and their accretions from the root surface, which would make nonsurgical therapy more efficient and effective.5 Furthermore, emerging new self-care devices may make patients’ oral hygiene more effective, thus preventing re-infection of treated periodontal pockets.6
With the quickening pace of advancement in clinical practice, many practitioners are left wondering how to best treat their patients. At meetings with dentists and dental hygienists, a frequent question is how other practitioners in their geographic area are treating their patients. This is another way of asking, “Am I doing all I can to help my patients?” Clinicians have an obligation to offer patients not just the newest products and techniques that may show statistically significant benefits, but also traditional methods proven over time to be clinically effective and cost-efficient. Evidence-based reviews are very helpful in this regard, particularly systematic reviews of quality research studies using pooled data subjected to meta-analysis.
With this in mind, the authors designed and executed a survey to determine the trends in nonsurgical periodontal treatment—both traditional and contemporary—that are commonly recommended and performed by dentists and dental hygienists in general practice in Davis, California.
Methods, Materials, and Results
The authors developed a 12-question survey and distributed it to general dentists and their dental hygienists to assess the practical standard of nonsurgical periodontal care offered to their patients. The surveys—along with an explanation of its purpose and instructions for its completion—were sent via e-mail to 15 dentists, who were asked to also distribute them to their dental hygiene staff. The survey asked respondents to identify the criteria used in determining periodontal treatment recommendations and the treatment modalities performed in their general practices. At least one follow-up telephone call was made to each office to ensure the survey had been received and to encourage its completion. To preserve anonymity, surveys were returned via e-mail or regular mail to an independent statistician, who abstracted the responses and computed descriptive statistics. Surveys were received from 29 dental hygienists and six dentists—83% and 17% of the total respondents, respectively. The survey results are presented in Table 1 through Table 4.
Diagnostics
With regard to diagnostics, respondents reported that they use pocket depth and bleeding on probing as the basis of their decision on when to initiate scaling and root planing (SRP) (Table 1). Some of the most revealing information obtained, however, came from the write-in option “Other.” There, among 17 individual reasons given for the decision to initiate SRP were radiographic bone loss, oral hygiene status, home care status, presence of subgingival calculus, and others, demonstrating that clinicians are using critical thinking and a sophisticated thought pattern, rather than a one-size fits all approach. No respondents mentioned plaque sampling or other diagnostic tests.7 Diagnostic tests, such as the use of microbiologic culture and sensitivity tests along with evaluation of patients’ gingival crevicular fluid and saliva, have been studied and may one day be commonly used by clinicians.8,9
Adjunctive Procedures
Answers concerning adjunctive procedures recommended along with subgingival SRP showed that anti-infective agents in several forms are being used concurrently with SRP (Table 2). Because periodontal diseases are initiated by oral pathogens—and some of the pathogens are tissue invasive—concurrent use of anti-infective agents has become a common protocol in clinical practice.4,10
Of the respondents, 71% indicated use of adjunctive local delivery of antibiotics concurrently with SRP. The two sustained or controlled-release locally delivered antibiotics used in the United States include: Arestin® (Orapharma Inc., www.arestin.com), which contains 1 mg of minocycline hydrochloride microspheres; and Atridox® (Zila Inc., www.zila.com), which contains doxycycline hyclate 10%.4,11 In a systematic review of locally delivered antibiotics used as adjuncts to SRP, Bonito and colleagues concluded that studies favored the use of minocycline over other agents.12 Their meta-analysis of eight studies using subgingival minocycline spheres showed, on average, only an additional 0.49-mm pocket depth reduction over the 1.5-mm reduction in pocket probing depth achieved by root planing alone. Bonito concluded that questions remained as to “whether such improvements, even if statistically significant, are clinically meaningful.”12
An original impetus for locally delivered, site-specific chemotherapeutics was to treat isolated defects on strategic teeth that were not good candidates for surgical intervention. In this way, high levels of antibiotics would be delivered to the needed area without resorting to systemic antibiotics for a specific area.
Greenstein suggested considering the number of sites to be treated before deciding on systemic antibiotics or minocycline microspheres. He stated that systemic drugs, when needed, can improve the efficacy of mechanical instrumentation at a nominal cost, no additional treatment time, and can provide antimicrobial therapy for reservoirs of reinfection (eg, saliva, tonsils).13 Two systematic reviews report that the adjunctive use of systemic antibiotics does appear to enhance the results of root planing, as measured by increased attachment level gains14,15 and greater pocket probing depth reduction.15 Because of significant variability in protocol methodologies in the studies available for meta-analysis, it was difficult to establish a definitive conclusion on which antibiotic is most effective.14
Among respondents, 71% reported using antimicrobial subgingival rinses at root planing. Although not harmful, chlorhexidine gluconate as a subgingival irrigant may not enhance the results of root planing.16 In the presence of blood products, the antimicrobial effect of chlorhexidine in the periodontal pocket is greatly reduced or eliminated.17
Adjunctive laser use during SRP was reported by 20% of respondents. In a recent review, Ishikawa et al5 stated that the Er:YAG laser seems to provide the most suitable characteristics for various types of periodontal treatment. In particular, it shows the most promise for root surface treatment and is safe and efficient for use in periodontal bone surgery when used with concomitant water irrigation.
Oral Hygiene Devices
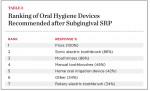
The most popular oral hygiene devices recommended after SRP were floss, mouthrinses, and sonic electric toothbrushes (Table 3). On the survey, many of those surveyed mentioned the use of proxy brushes and rubber-tip stimulators in the “Other” box. Home oral irrigation was recommended by 42% of respondents. Home oral irrigation devices are statistically and clinically effective at reducing gingival inflammation and periodontal disease activity.18-20 Daily home oral irrigation with water has been shown to be as effective at reducing gingival inflammation as rinsing with 0.12% chlorhexidine twice daily.19 In patients with adequate dexterity, water irrigation may be a cost-effective alternative to prescription mouthrinses with fewer side effects.
Periodontal Maintenance
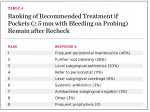
Forty-six percent of respondents indicated that they would place patients with unresolved deep pocketing (5 mm or greater) and areas of active inflammation after initial SRP on frequent maintenance; and 20% would use subgingival antibiotics (Table 4). If calculus is still present on the root surface, further root planing can be helpful, but periodontal maintenance or further subgingival antibiotic placement alone is unlikely to resolve this problem. Lang and associates have shown that pockets 5 mm or less that do not bleed on probing tend to be stable with regard to bone loss.21 Interestingly, only 11% of respondents would refer their patients to a periodontist if initial therapy did not produce a clinically stable situation. This may mean that clinicians are comfortable trying further root planing or frequent periodontal maintenance prior to referring to a periodontist. However, if active disease remains, placing a patient in a long-term “holding pattern” has the potential to endanger their periodontal health. After referral, periodontists can provide advanced nonsurgical instrumentation techniques and/or surgical therapy if indicated. Surgical therapy can provide visual access for root instrumentation, reduce pocket depths, regenerate lost periodontium, and is supported by evidence-based research.22-24
Recommended Method
More nonsurgical options for treating periodontal diseases exist today than ever before. However, removal of root accretions is still the recommended method. It has been practiced for centuries and was recommended almost 1,000 years ago in a medical encyclopedia by the Arab physician Abulcasis.25 Today there is a large volume of evidence-based literature supporting the efficacy of root planing and removal of root accretions.26,27 It is important that clinicians use evidence-based research as much as possible when deciding when to initiate interventional nonsurgical therapy, which nonsurgical adjuncts to use, and what home care devices and regimens to recommend to their patients.
Conclusion
The authors determined from their small survey that, although most clinicians use widely accepted criteria in their diagnosis and treatment recommendations, they may have different opinions regarding certain aspects of periodontal care. With all of the new treatment technologies being introduced, and as general dentists and dental hygienists update their periodontal treatment protocols, identifying evidence-based approaches for optimal care of their patients is as important as ever.
Acknowledgment
The authors would like to thank Kevin Grayson, DVM, PhD, for his statistical analysis of the collected data.
References
1. Armitage GC, Research, Science and Therapy Committee, American Academy of Periodontology. Diagnosis of periodontal diseases. J Periodontol. 2003;74(8):1237-1247.
2. Lanning SK, Best AM, Hunt RJ. Periodontal services rendered by general practitioners. J Periodontol. 2007;78(5):823-832.
3. Amano A. Host-parasite interactions in periodontitis: microbial pathogenicity and innate immunity. Periodontol 2000. 2010;54(1):9-14.
4. Jolkovsky DL, Ciancio SG. Chemotherapeutic agents. In: Newman MG, Takei HH, Klokkevold PK, Carranza FA. Carranza’s Clinical Periodontology. St. Louis, MO: Elsevier; 2006:798-812.
5. Ishikawa I, Aoki A, Takasaki AA, et al. Application of lasers in periodontics: true innovation or myth? Periodontol 2000. 2009;50:90-126.
6. Van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55(1):104-123.
7. Lamster IB. In-office diagnostic tests and their role in supportive periodontal treatment. Periodontol 2000. 1996;12:49-55.
8. Shaddox LM, Walker C. Microbial testing in periodontics: value, limitations and future directions. Periodontol 2000. 2009;50:25-38.
9. Giannobile WV, Beikler T, Kinney J, et al. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52-64.
10. Saglie FR, Carranza FA Jr, Newman MG. The presence of bacteria within the oral epithelium in periodontal disease. I. A scanning and transmission electron microscopic study. J Periodontol. 1985;56(10):618-624.
11. American Academy of Periodontology Statement on Local Delivery of Sustained or Controlled Release Antimicrobials as Adjunctive Therapy in the Treatment of Periodontitis. J Periodontol. 2006;77(8):1458.
12. Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol. 2005;76(8):1227-1236.
13. Greenstein G. The efficacy of minocycline microspheres in the treatment of chronic periodontitis: statistical vs. clinical significance. Compend Contin Educ Dent. 2003;24(2):121-133.
14. Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8(1):115-181.
15. Herrera D, Sanz M, Jepsen S, et al. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29(suppl 3):136-159.
16. Quirynen M, Mongardini C, De Soete M, et al. The role of chlorhexidine in the one-stage full mouth disinfection treatment of patients with advanced adult periodontitis: long-term clinical and microbiological observations. J Clin Periodontol. 2000;27(8):578-589.
17. Oosterwaal PJ, Mikx FH, Van den Brink ME, Renggli HH. Bactericidal concentrations of chlorhexidine digluconate, amine fluoride gel and stannous fluoride gel for subgingival bacterial tested in serum at short contact times. J Periodontol Res.1989;24(2):155-160.
18. Greenstein G, Research, Science and Therapy Committee of the American Academy of Periodontology. The role of supra- and subgingival irrigation in the treatment of periodontal diseases. J Periodontol. 2005;76(11):2015-2027.
19. Flemmig TF, Newman MG, Doherty FM, et al. Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. I. 6 month clinical observations. J Periodontol. 1990;61(2):112-117.
20. Husseini A, Slot DE, Van der Weijden GA. The efficacy of oral irrigation in addition to a toothbrush on plaque and the clinical parameters of periodontal inflammation: a systematic review. Int J of Dent Hyg. 2008;6(4):304-314.
21. Lang NP, Adler R, Joss A, Nyman S. The absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17(10):714-721.
22. Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol. 2001;72(12):1790-1800.
23. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8(1):227-265.
24. Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8(1):266-302.
25. Shklar G. Arabian Medicine. In: Carranza FA, Shklar G, Williams RC. History of Periodontology. Hanover Park, IL: Quintessence Books; 2003:37-42.
26. Van der Weijden GA, Timmerman MF. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(suppl 3):55-71.
27. Eberhard J, Jepsen S, Jervøe-Storm PM, et al. Full-mouth disinfection for the treatment of adult chronic periodontitis. Cochrane Database Syst Rev. 2008;(1):CD004622.
About the Authors
David L. Jolkovsky, DMD, MS
Lecturer
School of Dentistry University of California Los Angeles
Los Angeles, California
Diplomate, the American Board of Periodontology
Private Practice
Davis, California
Samer S. Alassaad, DDS
Private Practice
Davis, California
Lee A. Grayson, RDH, BS
Private Practice
Davis, California