Small-Diameter Implants and Crestal Bone Retention
Calcium phosphate helps to induce osteoblasts at the implant surface and reduce the catabolic phase of bone.
Since the introduction of dental implants, clinicians have developed strategies to accelerate healing and shorten the time to final prosthetic reconstruction. These strategies have included modifying implant macro-architecture, micro-architecture, and surface chemistry.1 While the first surface treatment used acid-etching to increase surface area, the earliest attempt to incorporate a bone-like surface treatment employed a plasma-sprayed hydroxyapatite (HA) appositional coating to improve the quality of osseointegration.2 Although long-term results on HA-coated implants have been inconclusive, additional studies using calcium-phosphate materials have been conducted with more definitive outcomes.
The particular focus of this article is a surface that employs a molecular-impregnated calcium-phosphate modification known as Ossean® (Intra-Lock System of Canada, www.intra-lock.ca). This surface is biologically active in the sense that the surface chemistry mitigates the catabolic—ie, breakdown—phase of bone leading to acceleration of anabolic bone bonding. This phenomenon is particularly interesting in cases of immediate loading—especially for small-diameter implants—or in cases of standard-diameter implant placement within an extraction site or areas where initial bone-to-implant contact is limited.
When evaluating an implant system, the clinician should focus on two things. First to be examined is architecture—length, diameter, whether it is parallel or tapered, and the aggressiveness of the thread profile. Second to be considered is the implant surface, which can vary from a simple superficial etch to the application of plasma-sprayed hydroxyapatite coatings or, more recently, impregnation at the molecular level with calcium phosphate. All of these surface treatments are presented as “bioactive,” as they will all modify the host’s biologic response. The difference lies in knowing how this response differs qualitatively and the relationship to the type of surface treatment employed. In view of the emerging outcomes pursuant to these various technical modifications, the authors have concluded that etched-surface implants have limited biologic effects when compared to nanostructured calcium-phosphate–impregnated implants.3
One of the least-studied parameters in oral implantology is the relationship between the catabolic and anabolic phases of bone. It has been demonstrated that, during the first 1 to 3 weeks after implant placement, the shear strength between bone and implant is weaker than it is immediately after insertion.4 This phase is characterized by bone microfracture, hypoxia-induced inflammation, acidic pH, production of proteases such as collagenase, and osteoclastic activity.
Many factors influence the catabolic phase of bone healing. The first identified is that of bone compression occurring when torqueing implants. This compression generates microfractures, which can extend laterally from the implant body.5 It has been demonstrated that if the torque exceeds 55 Ncm—a torque higher than normal physiologic stress—it will contribute to the catabolic phase of the bone. Stress applied to implants during this time period, as is often the case with small-diameter one-piece implants, may have deleterious consequences, as the woven bone that forms is less capable of withstanding stress of function. Only after completion of this remodeling activity can the bone enter its regenerative anabolic phase.
Another important part of the inflammatory cascade is a change in pH in the bone surrounding the implant. Osteoblast metabolic activity and genetic expression are modulated by cellular pH level. It has now been shown that even nominal lowering of pH can completely suppress osteoblast activity by shutting down the early response EGR-1 gene.6 At this time, type III human collagen, which acts as a scaffold for the development of a provisional matrix, will not be produced. Calcium phosphate acts as a buffering solution, keeping the pH closer to physiological neutral and decreasing osteoclastic activity. Because it is well known that the catabolic phase is at its most active during the first 1 to 3 weeks and is characterized by a lower pH, it is easy to understand that any strategy aiming at minimizing this process may significantly increase early bone bonding.
The anabolic phase of bone is characterized by angiogenesis, osteoblastic activity, production of type III human collagen, formation of a provisional matrix, and remodeling of woven bone into mature, stress-bearing lamellar bone.7 It is, therefore, easy to deduce that reduction of the catabolic phase or acceleration of the anabolic phase of bone would be desirable sequelae and may contribute to implant success and retention of crestal bone levels.
Free ionic calcium is responsible, at the mitochondrial level, for early protein production. Measurement of the production of mitogen-activated proteins (MAP kinases) is a means to calculate the relative rates of cell metabolism. When calcium levels are at ideal levels, osteoblast metabolism can be ramped up by as much as 500%.8 Collagen production is substantially increased, leading to faster bone production. Free ionic calcium also produces a variety of biological effects, including bone-derived cell lines. It can stimulate stem cell differentiation into osteogenic lines.9 One of the more interesting facts about calcium phosphate is its osteoinductive power even in the absence of stem cells, bone morphogenetic proteins, or osteoinductive cytokines.10
Another important effect of free ionic calcium is that it stimulates the release of parathyroid hormone-related protein (PTHrp), which plays an important role in bone densification. This phenomenon can be measured within 60 minutes after placement of an implant with a calcium-phosphate substrate.11
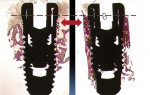
A study that focused on inserting two implants that were to eventually be removed—one with the Ossean surface and the other with a simple double-acid-etched surface—concluded that there was a significantly higher bone-to-implant contact and osteocyte index for the test surface (Ossean) in comparison with the control surface (acid-etched).12 Histologic samples showed that the density was much higher after 2 months for the Ossean surface implant and, even more surprising, higher crestal bone reformation with the Ossean surface in comparison with the double acid-etched surface. Figure 1 shows two implants inserted in a patient. The lack of contact between bone and implant for the control surface is evident and impressive. The number of osteocytes, which was also evaluated in this study, was 30% higher for the Ossean surface implant.
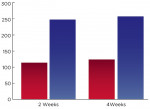
Another study focused on implant insertion in an animal model with reverse torque pullout.13 Focusing on the same two types of implants differing only by their surface, the torque needed to remove them was evaluated at 2 and 4 weeks after insertion. Once again, results were notable; twice as much torque was needed to remove Ossean surface implants in comparison with acid-etched implants (Figure 2). It is important to note that the timeframe in which this entire study was completed was during the catabolic phase of bone remodeling.
Clinical Cases
It is also interesting to look at some clinical results to see if the laboratory or animal research results can be corroborated with similar clinical successes.
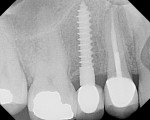
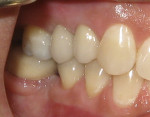
The first case is a 3-mm Milo implant (one-piece implant) with the Ossean surface from Intra-Lock International that was inserted in 2006 (Figure 3). Three weeks later, it was fitted with a final fixed crown. Results assessed 5 years after insertion showed that clear bone with better density was observable without crestal bone loss (Figure 4).
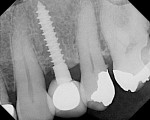
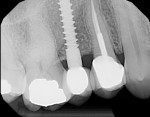
In a case that involved replacement of tooth No. 14, another Milo 3-mm implant with the Ossean surface was placed in 2009 with 45 Ncm of torque; the final crown was cemented 2 weeks later. As is shown in Figure 5, the bone was not very dense at the crestal level at the time of the insertion. Figure 6 is the intraoral picture after cementation of the crown.
After 2 years, a radiograph (Figure 7) showed better dense bone at the crestal level, as well as growth of bone over the collar level. This clearly demonstrates the effects of calcium-phosphate impregnation on bone density and crestal bone levels.
Those clinical cases demonstrate the positive effect of surfaces such as Ossean on early bone reaction, not only at the implant body level but also at the collar level, a site where bone loss that occurred was traditionally attributed to biological recovery and establishment of biologic width.
Conclusion
In light of these studies—whether in vitro, in animal studies, or in vivo—it is now evident that the chemical composition of the surface, calcium phosphate, plays an important role in inducing osteoblasts at the implant surface and reducing the catabolic phase of bone. The Ossean surface meets the criteria for a biologically active implant surface and raises the bar as to what can be expected of early bone healing around implants. It is clear that this is especially important for small-diameter implants in which it is desirable to preserve or increase the crestal bone quantity and density, but also desirable to achieve osseointegration as rapidly as possible.
Disclosure
The authors have a financial interest in Intra-Lock System of Canada.
References
1. Lee SC, Song WS. Histomorphometric and removal torque values comparison of rough surface titanium implants. J Korean Assoc Maxillofac Plast Reconstr Surg. 2001;23(5):396-405.
2. MacDonald DE, Betts F, Stranick M, et al. Physicochemical study of plasma-sprayed hydroxyapatite-coated implants in humans. J Biomed Mater Res. 2001;54(4):480-490.
3. Coelho P, Freire J, Coelho A, et al. Nanothickness bioceramic coatings: Improving the host response to surgical implants. In: Leipsch D, ed. World Congress of Biomechanics Conference Proceedings. Munich: Medimont. 2006;253-258.
4. Albreksson T, Johansson C. Quantified bone tissue reactions to various metallic materials with reference to the so-called osseointegration concept. In: Davies JE, ed. The Bone-Biomaterial Interface. Toronto: University of Toronto Press. 1991;357-363.
5. Guimaraes MV, et al. Early bone healing around different implant bulk designs and surgical techniques: A study in dogs. Clin Implant Dent Relat Res. 2010;12(3):202-208.
6. Schilling AF, Linhart W, Filke S, et al. Resorbability of bone substitute biomaterials by human osteoclasts. www.sciencedirect.com/science/journal/01429612">Biomaterials. 2004;25(18):
3963-3972.
7. Coimbra M, Salles M, Yoshimoto, M, et al. Physico/Chemical Characterization, In Vitro, and In Vivo Evaluation of Hydroxyapatite/PLGA Composite and Tricalcium Phosphate Particulate Grafting Materials. TITANIUM: The International Journal of Dental Implants & Biomaterials. 2009;1(1):16-28.
8. Bucci V, Cassinelli C, Coelho P, et al. Effect of titanium implant surface roughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(2):217-224.
9. Jaiswal RK, Jaiswal N, Bruder SP, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645-9652.
10. Chai YC, Roberts SJ, Schrooten J, Luyten FP. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng Part A. 2011;17(7-8):1083-1097.
11. Tfelt-Hansen J, MacLeod RJ, Chattopadhyay N, et al. Calcium-sensing receptor stimulates PTHrP release by pathways dependent on PKC, p38 MAPK, JNK, and ERK1/2 in H-500 cells. Am J Physiol Endocrinol Metab. 2003;285(2):E329-E337.
12. Shibli JA, Grassi S, Piatelli A, et al. Histomorphometric evaluation of bioceramic molecular impregnated and dual acid etched implant surfaces in the human posterior maxilla. Clin Implant Dent Relat Res. 2012;12
(4)281-288.
13. Marin C, Granato R, Suzuki M, et al. Removal torque and histomorphometric evaluation of bioceramic grit-blasted/acid-etched and dual acid-etched implant surfaces. An experimental study in dogs. J Periodontol. 2008;79(10):1942-1949.
About the Authors
Bruno Lemay, DMD
Founder
CMI Institute
Private Practice
Cathedral City, California
Robert J. Miller, MA, DDS
Chairman, Department of Oral Implantology
Atlantic Coast Dental Research Clinic
Lake Worth, Florida