CERAMIR® Crown&Bridge
A bioactive hybrid acid-base reaction cement for prosthodontic applications.
CERAMIR® Crown &Bridge is an acid-base reaction cement and constitutes a paradigm shift from resin-based and resin-modified glass ionomer and adhesive resin cements. Ceramir C&B is not a resin cement, yet it has physical properties that equal or exceed the aforementioned cements. A most important property of any cement for long-term success is biocompatibility. Post-cementation hypersensitivity is a frequently occurring clinical problem and can result in further complications requiring additional chairside time and expense.
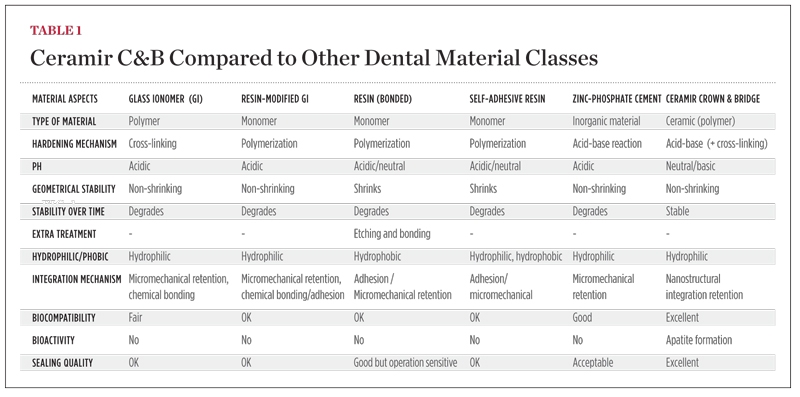
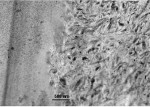
Ceramir C&B is a water-based hybrid composition comprised of calcium aluminate and glass-ionomer components (GIC) that are mixed with a liquid. The setting mechanism of Ceramir C&B is a combination of a glass-ionomer reaction and an acid-base reaction of the type occurring in hydraulic cements. After setting, the material is slightly acidic and has a pH ~5. After 40 minutes the pH is already neutral, and after 2 to 3 hours it reaches a basic pH of ~8.5. This means that the fully hardened material is basic and stays basic throughout its service unlike other acid-base reaction cements, such as zinc phosphate, polycarboxylate, and glass-ionomer cements. The basic property is a major contributor to its biocompatibility and probably one of the most important prerequisites for the material to be bioactive, ie, creating hydroxyapatite on its surface when in contact with phosphate-containing solutions.1 The material also produces an excess of Ca2+ ions, which also contribute to its bioactivity and to possible remineralization of surrounding tooth structure, more significantly of enamel than dentin (Figure 1 and Figure 2). The incorporation of calcium aluminate fixes the GIC structure and hinders the glass ionomer from continuously leaking over time. Ceramir C&B has an initial fluoride release comparable to a glass ionomer, which tapers off over time. Unique properties such as apatite formation develop quickly and continue to be active. Other parameters that determine uniqueness are listed in Table 1. For information on biocompatibility tests, click here www.ceramir.se/web/Infomaterial_text_pdf_1.
Bioactivity
CaAl is a novel material that exhibits bioactivity properties and has the necessary mechanical properties to function as a dental restorative material.2,3 Bioactivity of a ceramic material is a surface property that establishes a bond between the material and living tissues without fibrous encapsulation. The most important part of this mechanism is the ability of mineralization of calcium phosphates on a material's surface in vivo. The mechanisms behind this and how the hydroxyapatite (HA) surface layer is formed has been reported elsewhere.2,4
Ceramir C&B has promising applications for the majority of indirectly fabricated restorations that require cementation. Other formulas based on the CaAl platform have potential applications in dentistry and are currently being developed.
This article was written by Cornelis H. Pameijer DMD, DSc, PhD, Professor Emeritus at University of Connecticut.
References
1. Lööf J, Svahn F, Jarmar T, et al. A comparative study of the bioactivity of three materials for dental materials. Dent Mater. 2007;24(5): 653-659.
2. Lööf, Engqvist H, Ahnfelt NO, et al. Mechanical properties of a permanent dental restorative material based on calcium aluminate. J Mater Sci: Mater Med. 2003;14(12):1033-1037.
3. Lööf J, Engqvist H, Gómez-Ortega G, et al. Mechanical property aspects of a biomaterial based restorative system. Key Eng Mater. 2005;284-286:741-744.
4. Jefferies SR, Pameijer CH, Appleby D, et al. One-year clinical performance and post-operative sensitivity of a new bioactive dental luting cement—a prospective clinical pilot study. Swed Dent J. 2009;33(4):193-199.
For more information, contact:
Doxa Dental AB
www.ceramir.se
Disclaimer
The preceding material was provided by the manufacturer. The statements and opinions contained therein are solely those of the manufacturer and not of the editors, publisher, or the Editorial Board of Inside Dentistry.