Guided Wound Healing
Steven I. Present, DMD
Case presentation demonstrates transmucosal development in the esthetic zone.
The development and appearance of the transmucosal region as the tooth emerges into the oral cavity can be the difference between treatment failure and success. To optimize the chances for a successful treatment outcome, dentists must base their clinical protocols on sound biologic principles with an understanding of the effects their procedures have on wound healing and tissue repair.
The emergence profile is defined as the subgingival contours of the restoration as it emerges through the soft tissues to form the clinical crown. It supports the pericoronal soft tissues (free gingival margins and papillae) and is a critical factor in determining esthetics, and, ultimately, the difference between the success and failure of the restorations. A primary goal in the development of the soft tissue contours is to have predictability and long-term stability. To achieve this, dentists must understand and manage the biology of wound healing.
Through the evolution of surface technology from a smooth machined surface to the hydrophilic SLActive® (Straumann, https://www.straumann.us) the time of osseointegration can be reduced to a significant degree. This, however, is not the case with soft tissue. When clinicians discuss transmucosal development, what they are really trying to accomplish is guiding the healing of the pericoronal tissues to the desired shape and dimensions for proper support and to mimic as closely as possible the pericoronal tissues of the contralateral tooth.1,2
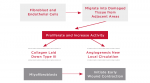
Healing can be defined as the body's replacement of destroyed tissue by living tissue. There are basically two components of soft tissue healing: regeneration and repair. With regeneration, specialized tissue is replaced by the proliferation of the surrounding undamaged, specialized cells. With repair, lost or damaged tissue is replaced by granulation tissue, which matures to form a scar. This is the type of healing clinicians try to manipulate around dental implants. As illustrated in Figure 1, there are four phases of tissue repair: bleeding, inflammation, proliferation, and remodeling, which are not mutually exclusive and can overlap.3,4
Bleeding Phase
The following points are the major characteristics of the bleeding phase:
Bleeding Phase
• Relatively short-lived.
• Normal bleeding time will vary with the nature of the injury and the tissue. The more vascular the tissues, the longer they will bleed.
• Normally, the time between the injury to the end of bleeding is a few hours.
Inflammatory Phase
The following points are the major characteristics of the inflammatory phase (Figure 2:
• Essential for tissue repair.
• Rapid onset (few hours) and increases in magnitude for 2 to 3 days before gradually resolving over a few weeks.
• A complex, chemically mediated amplification cascade is responsible for the initiation and control of the inflammatory reaction.
• Vascular and cellular cascade are the two essential elements. They occur parallel to each other and are interlinked.
Because of histamine, vasodilatation causes increased blood flow and complement cascade components C3 and C5. White blood cells migrate, platelets adhere to vessel walls, and endothelial cells swell, resulting in increased vasopermeability combined with increased blood flow, which results in increased exudates, including plasma proteins. This is because histamine, serotonin (5-HT), bradykinin, and leukotrienes are all potentiated by prostaglandins. Mast cells release hyaluronic acid, which binds with the exudate to create a gel that limits flow. Cellular components include early neutrophil emigration from vessels, followed by monocytes, lymphocytes, eosinophils, and basophils. Polymorphonuclear neutrophils act as early debriders. An end product of phagocytosis is lactic acid-a stimulus of proliferation, the next sequence.
Proliferation Phase
The following points are the major characteristics of the proliferation phase (Figure 3.
• Involves generation of repair material.
• Rapid onset, 24 to 48 hours.
• Peak activity reached in 2 to 3 weeks.
• Decreases over several months.
• Two fundamental processes: fibroplasia and angiogenesis.
• Chemical mediators, ie, macrophage-derived growth factors, platelet-derived growth factors, lactic acid, fibroblast growth factor.
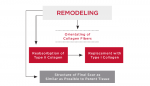
Remodeling Phase
These are the major characteristics of the remodeling phase (Figure 4:
• Primarily involves collagen and the extracellular matrix.
• With maturity, collagen becomes more oriented in line with local stress.
Type III collagen, which is fine, weak, and highly cellular (it is the collagen of granulation tissue and produced by young fibroblasts) is converted to type I collagen, which is more cross-linked with greater tensile strength and, therefore, more stable.5
Although wound healing involves the four stages as previously listed, dentists must be aware of the factors that can have an adverse influence and are known to cause its delay. General factors include age, protein deficiency, low vitamin C levels, use of steroids and NSAIDs, and low temperature. Local factors include poor blood supply and ischemia, adhesion to bone or other underlying tissue, continued inflammation, drying of the wound, and excessive movement.
Because there cannot be proper wound healing without inflammation and the production of prostaglandins plays an essential role, clinicians must ask, does the use of NSAIDs affect wound healing?6 Animal studies indicate a delay in the repair of damaged tissue including decreased collagen concentration, decreased bone healing, and decreased bone-to-implant contact. However, these studies are small and equivocal. Large prospective studies are needed. Another question that has to be investigated is if a patient is only on an NSAID for a short period of time is there a negative clinical effect on wound healing? A corollary to this question would be is there anything that can be done to accelerate soft tissue healing? One of the main initiators of both hard and soft tissue is recombinate-human platelet-derived growth factor BB (rh-PDGF BB). It is a potent activator of mesenchymal cells, and it stimulates chemotaxis, proliferation, and new gene expression in monocytes, macrophages, and fibroblasts. Incision wound studies have shown that rh-PDGF BB augmented wound strength by 50% and accelerated wound closure by 30% over the first 3 weeks.7-9 Additional animal and human studies show dramatically enhanced re-epithelization and prevented wound contracture.10,11 For immediate temporization, it may be prudent to use rh-PDGF BB.12
For an implant-supported crown to be esthetic, it must be in harmony with the peri-oral facial structures of the patient and imitate the natural appearance of the missing dental unit(s) in color, form, texture size, and optical properties. This all depends on proper submucosal positioning in all three dimensions, long-term stability of esthetic and peri-implant soft tissue contours, and symmetry of clinical crown volumes between the implant site and the contralateral teeth.
As they emerge into the oral cavity, teeth in the anterior maxilla have a triangular cross-sectional shape; however, dental implants are circular. To establish the proper emergence profile, support the peri-implant soft tissue, and optimize treatment outcomes, the use of provisional restorations are necessary to guide the healing and shape the tissues prior to the delivery of the definitive restorations. However, how an immediate site is managed differs from a site that is already healed and matured. When an immediate provisional is fabricated, the subgingival contours should be slightly concave to encourage excessive tissue development. With a matured healed site, the proliferation phase of the healing process is completed; therefore, the provisional restorations can be contoured as necessary to develop the final desired contours.
As shown by Raghavendra's Implant Stability Curve (Figure 5 it is important for provisional restorations to be placed within the first week of implant placement because of the dramatic drop off of primary stability, and left alone for at least 4 weeks.13 This also coincides with the completion of the proliferation phase (Figure 6. At this time the clinician can start to adjust the contours of the provisional restorations. If final impressions are made prior to the near-completion of the remodeling phase, the peri-implant tissues will be immature and the clinician will not be in control of the final results and the long-term stability of the final restorations.
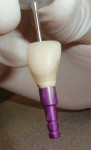
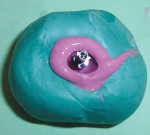
Once the final tissue contours and the transmucosal zone have been established, that information must be communicated to the laboratory technician. This is accomplished with custom impression copings.
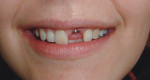
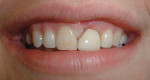
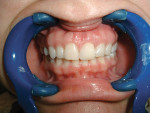
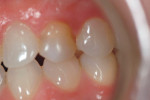
Case 1: Healed Site
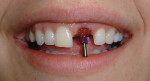
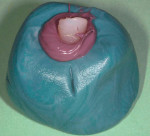
A 27-year-old woman was referred to the author for the restoration of tooth No. 9 (Figure 7. The implant in that site was placed by her periodontist in Illinois approximately 5 to 6 months before she moved to the Philadelphia area. Her medical and dental history was unremarkable; she was wearing a Hawley retainer with a tooth as a provisional restoration. There was a mesial-distal size discrepancy; the edentulous space in No. 9 was slightly greater than in No. 8. It was decided to make up the difference with the placement of a direct composite on tooth No. 10. Because this was a matured healed site, a screw-retained provisional was fabricated with the estimated ideal contours to sculpt the transmucosal zone (Figure 8 and Figure 9.
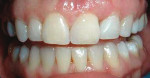
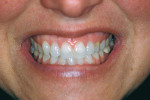
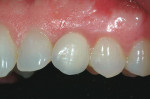
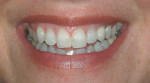
She was recalled every 2 weeks to evaluate the soft tissue architecture. Adjustments to the provisional were made as necessary to establish the desired shape of the free gingival margin and interdental papillae. Once the desired contours were established with the provisional, an additional 2 weeks was allowed before the final impression was made. At the 3-year follow-up she has shown excellent stability of the transmucosal zone while maintaining the desired height of the free gingival margin and papillae (Figure 10, 11, 12, 13.
Case 2: Immediate Site
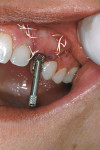
A 35-year-old woman presented with a failing retained left primary canine. Tooth No. 11 was congenitally missing (Figure 14. Her medical and dental health was unremarkable except for the replacement of tooth No. 30 with a Straumann tissue-level implant in 1998. The patient refused orthodontic therapy to align the anterior dentition. A surgical stent was fabricated from a diagnostic wax-up for the surgical placement of the implant in the proper three-dimensional position. Immediately after implant placement surgery, the patient reported to the author's office for the fabrication of a screw-retained provisional. The provisional was under-contoured to develop a slight excess of tissue in the transmucosal zone in order to develop a slight excess of peri-implant soft tissue. The provisional was coated with Polysporin® ointment upon placement and chlorhexidine 0.12% rinse was prescribed. A screw-retained provisional was used to avoid the possibility of any residual cement, which can compromise healing; in addition, it would facilitate the future fabrication of the custom impression coping (Figure 15 and Figure 16. After approximately 4 weeks, to give adequate time for the near-completion of the proliferation phase of healing, the patient was recalled to begin adjusting the contours of the provisional to develop the ideal emergence profile to support free gingival margins and interdental papilla (Figure 17.
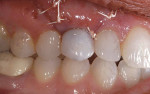
After approximately 11 weeks of additional healing (15 weeks post-surgery), the healing process had progressed to the point of making the final impression. A custom impression coping was fabricated and the impression was made (Figure 18. The 2-year follow-up shows good maintenance of the free gingival margin and interdental papilla. The free gingival margin is not at the ideal apical position. Upon questioning the surgeon, he stated that the surgical stent was not used. Although the implant was placed in a good mesial-distal and facial-palatal position, it was not placed far enough apically to position the facial-gingival margin at the same level as tooth No. 11. However, the patient has been satisfied with the final results (Figure 18, 19, 20.
Discussion
Predictability and stability are the goals of implant-supported restorations. To achieve this goal, clinicians must give the peri-implant tissue adequate time to develop and mature. Therefore, clinicians must observe the stages of wound healing and time their restorative procedures accordingly.
Disclosure
Dr. Present has received honoraria from Straumann.
Editor's Note
The author originally presented the information contained in this article at the ITI US Congress Meeting held in Scottsdale, Arizona, in 2009.
References
1. Belser U. ITI Treatment Guide. Hanover Park, IL: Quintessence; 2007;1:38-46.
2. Higginbottom F, Belser U, Jones JD, Keith SE. Prosthetic management of implants in the esthetic zone. Int J Oral Maxillofac Implants. 2004;19(Suppl):62-74.
3. Walter JB, Israel MS. General Pathology. Edinburgh: Churchill Livingstone. 1987;6:117-129.
4. Ryan GB, Manjo G. Acute inflammation: A review. Am J Pathol. 1977;86(1):184-276.
5. Hardy MA. The biology of scar formation. Phys Ther. 1989;69(12):1014-1024.
6. Iino G, Nishimura K, Omura K, Kasagai S. Effects of prostaglandin E1 application on rat incisal sockets. Int J Oral Maxillofac Implants. 2008;23(5):835-840.
7. Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative anti-inflammatory and immunomodulating therapy on surgical wound healing. Pharmocotherapy. 2005;25(11):1560.
8. Saba AA, Freedman BM, Gaffield JW, et al. Topical platelet-derived growth factor enhances wound closure in the absence of wound contraction: an experimental and clinical study. Ann Plast Surg. 2002;49(1):62-66.
9. Pablos AB, Ramalho SA, Koenig B Jr, et al. Effects of melcicam and diclofenac sodium on peri-implant bone healing in rats. J Periodontol. 2008;79(2):300-306.
10. Ibara YL. Histometric study of socket healing after tooth extraction in rats. Braz Dent J. 2002;(13)2:92-96.
11. Yugoshi LI, Sala MA, Brentegani LG, Lamano Carvalho TL. Histometric study of socket healing after tooth extraction in rats treated with diclofenic. Braz Dent J. 2002;13(2):92-96.
12. Hom DB, Thatcher G, Tibesar R. Growth factor therapy to improve soft tissue healing. Facial Plast Surg. 2002;18(1):41-52.
13. Raghavendra S. Early wound healing adjacent to endosseous dental implants: A review of the literature. Int J Oral Maxillofac Implants. 2005;20(3):425-431.
About the Author
Steven I. Present, DMD
Clinical Associate Professor
Temple University
Kornberg School of Dentistry
Philadelphia, Pennsylvania
Faculty
Manor College
Jenkintown, Pennsylvania
Fellow
International Team for Implantology
Basel, Switzerland