Perio Tray®
Prescription device places solutions in gingival crevices and periodontal pockets.
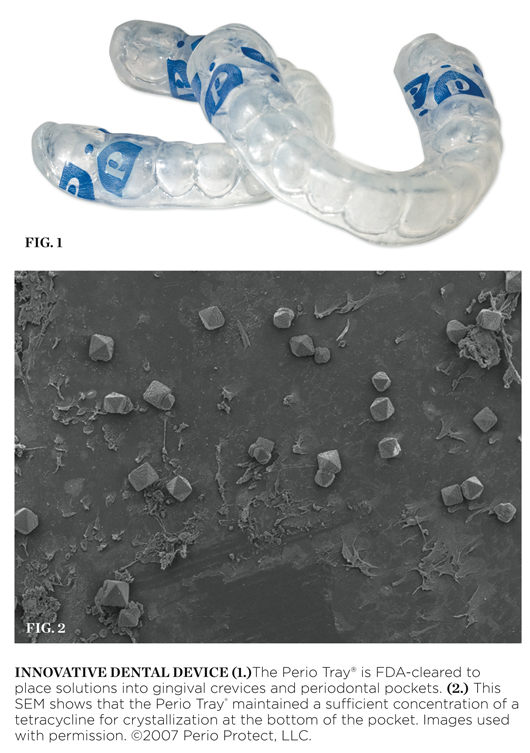
The Perio Tray® is a prescription device cleared by the Food and Drug Administration (FDA) in 2004 for the intended use to place solutions of the clinician’s choice into gingival crevices or periodontal pockets (Figure 1). The choice of solution, the frequency of use, and the time the Perio Tray is to be in place are a part of the practice of dentistry and are determined by the treating dentist based on individual patient needs.
In addition to data submitted for FDA clearance, an independent analysis completed in 2006 at the University of Southern California’s Center for Biofilms demonstrates the efficacy of medicament placement into periodontal pockets via the Perio Tray. In this study, small sterile polycarbonate carriers were placed into three 6-mm pockets (teeth Nos. 14 and 15) for 48 hours to allow biofilm to grow on the carrier surface. The carriers were then extracted and analyzed with a scanning electron microscope. Throughout the course of the study, the carriers were inserted and extracted to analyze the changes in the microbial ecology of the pockets after solutions were placed into the pockets with the Perio Tray. In the second phase of the study, a subclinical dosage of tetracycline (one drop) was placed into the Perio Tray with an eyedropper in the location of the pockets. The Perio Tray was then worn by the patient to place the tetracycline into the pockets. This protocol was repeated 4 times a day for 20 minutes for 5 days. The carriers were then inserted and removed 2 days later. Upon extraction of the carriers, tetracycline crystals were observed throughout the surface of the carrier, including along the bottom portion of the carrier, indicating that the Perio Tray placement of this solution remained at sufficient concentration for crystallization at the bottom of the pocket. Images of the crystals were captured and analyzed with a scanning electron microscope (Figure 2).1
In order to place solutions into periodontal pockets, a Perio Tray must be customized to fit each patient precisely. The first step to a precise fit is taking a hydrocolloid alginate impression that includes the gingival margin around each tooth. Regular alginate materials can be used, but when using time- and temperature-sensitive impression materials, it often takes two people to mix the materials, place the materials around the teeth, load the impression tray, and insert the tray in a timely manner. When the impression material has set and is removed, it must be inspected for distortions, effects, or voids.
If the impression is good and has sufficient gum exposure, a model should be poured with appropriate stone. A well-poured model is one of the most critical aspects of fabricating a proper Perio Tray. Errors in the models can impede and delay production or, in some cases, even prevent it, forcing the patient to return to the office for another impression. Some dental offices prefer to send the impression to the dental laboratory, in which case the laboratory will pour the model and custom fabricate the Perio Tray.
In addition to a good model, Perio Tray fabrication requires information about the state of the patient’s gingival crevices and periodontal pockets. If the gingival tissues are inflamed or bleeding or if the periodontal pocket measures more than 3 mm, this information should be sent to the dental laboratory so that the Perio Tray will be fabricated to meet the individual conditions of each patient for the most comfortable and effective placement of the doctor-selected solution into the crevice or pocket. If the conditions of the crevice or pocket change over time—for example, decreased inflammation, decreased bleeding on probing, or reduced pocket depth—the precise fit of the Perio Tray will be compromised and a new Perio Tray® will be required to place solutions effectively into the crevices or pockets.
Because the Perio Tray has 510(k) clearance, it must be manufactured by a dental laboratory registered annually with the FDA. In the United States, the FDA-registered laboratories include Space Maintainers of the Midwest, Great Lakes Orthodontics, Ltd, Space Maintainers of California, and Space Maintainers of the Southeast.
Reference
1. Data presented at: 2007 IADR Meeting, abstract no. 1186: SEM Results of Periopathogenic Control with the Perio Protect Method.
For more information, contact:
Perio Protect, LLC
Phone: 866-693-3400
Web: https://www.periotray.com
Disclaimer
The preceding material was provided by the manufacturer. The statements and opinions contained therein are solely those of the manufacturer and not of the editors, publisher, or the Editorial Board of Inside Dentistry. The preceding is not a warranty, endorsement, or approval for the aforementioned products or services or their effectiveness, quality, or safety on the part of Inside Dentistry or AEGIS Communications. The publisher disclaims responsibility for any injury to persons or property resulting from any ideas or products referred to in the preceding material.