Breaking the “Generation” Gap
Using a revolutionary self-etching adhesive.
Over the past 60 years, the art and science of dental adhesive technology has exploded and forever changed the practice of clinical dentistry. Beginning in the 1950s with the introduction of acid-etching of enamel and the realization that the micromechanical retentive undercuts would permit the adaptation of hydrophobic resins, the pursuit of the ideal adhesive has been ongoing.1,2 It took less than a year after the acceptance of enamel bonding to prompt researchers and chemists to begin exploring the possibility of similar bonding mechanisms to hydrophilic dentin.3
Historically, the label of adhesives by “generation” began during the early 1960s with the introduction of bi-functional resins that possessed both hydrophilic and hydrophobic functional groups that in theory served as “coupling agents” to unite hydrophobic methacrylates to the moist dentin substrate.4 Originally, the smear layer (debris resulting from rotary instrumentation of the dentin surface) was considered the “biological Band-Aid” that must be preserved and the thought of removing it via acid-etching was nothing short of heresy. So the first three “generations” of adhesives focused on chemically bonding to either an intact smear layer, or a mildly conditioned smear layer via ionic bonds to calcium. Because the smear layer was weakly attached to the dentin surface, bond strengths were typically less than 3 MPa and resulted in early failure.
By the early 1980s, ground-breaking research on the hybridization of dentin by Nakabayashi and Pashley opened the door for the controversial concept of simultaneously etching both enamel and dentin with phosphoric acid and the complete removal of the dentin smear layer.6 This set the stage for what is now called the “fourth-generation” adhesives which advocated the concept of “total-etching” and the subsequent infiltration of polymerized acrylic monomers. The proper clinical technique of total-etch adhesives would soon be developed by Kanca and the necessity of “wet bonding” became established in the early 1990s.7 These fourth-generation materials, many of which have been improved and are still on the market today, involve numerous technically demanding steps to be exacted with precision in order to be effective. Fourth-generation systems are characterized by using a separate ambiphilic primer and hydrophobic adhesive, and many are considered “chemistry sets” that require multiple exacting steps and applications.
The desire to reduce the number of steps and simplify the clinical technique spawned the “fifth-generation,” single-bottle, total-etch adhesives. This generation of adhesives is characterized as having both the ambiphilic primer and hydrophobic resin adhesive in the same bottle. By combining primer and adhesive, these systems reduced the number of steps required, created less margin for clinical error, and immediately gained widespread acceptance. But because these systems still require a total-etch technique, the level of dentin moisture remains critical, and though some formulations boast a larger window of error through the varied use of solvents to reduce volatility and technique sensitivity, many practitioners continue to struggle with postoperative sensitivity. One of the latest “generations” of dental adhesives is the “sixth generation.” These products are characterized as being “self-etching” adhesives and do not require a separate phosphoric-acid etchant application. These products use an acidic primer or primer/adhesive that solvates the smear layer, removes calcium and phosphates, exposes collagen fibrils, and simultaneously diffuses hydrophilic primers and hydrophobic resins onto the demineralized dentin surface and into opened dentinal tubules.9 Because the smear layer is not removed and the etchant is not rinsed away leaving exposed collagen fibrils, the vulnerable phase between etching and primer application is avoided. The level of dentin moisture before and during the bonding process is much less critical and the margin of error is greatly reduced. Some systems, particularly single-bottle systems, reduce the number of steps required, resulting in less procedure time.
Until recently, all “sixth-generation” self-etching/priming adhesives contained volatile solvents that must be evaporated prior to light-curing in order to provide consistent bond strengths. One of the most common solvents in this generation of adhesives is water, which can be the most difficult to volatilize with air-drying. If residual water remains upon light-curing, bond strengths can be dramatically reduced and the permeability of the dentin/adhesive interface is questioned. Tay et al reported the phenomenon of postoperative transportation of water from the dentinal fluid through the semi-permeable barrier created by these hydrophilic systems, which may lead to premature hydrolysis and potentially failure of the bond and seal.10,11 The formation of “water trees,” found similarly in acrylics used as insulation around underground wire, suggest that an adhesive system that does not use hydrophilic solvents such as water may prove advantageous.
Introducing Bond-1® SF Solvent-Free SE Adhesive
Recently, a new solvent-free, self-etching, single-bottled system has been introduced by Pentron Clinical Technologies (https://www.pentron.com). Bond-1 SFSE Adhesive uses a proprietary formula that eliminates the need for commonly used solvents such as acetone, alcohol, and water, and not only reduces the number of application steps, but removes the ambiguity of air-volatilizing residual solvents prior to light-curing. Because residual solvents can reduce bond strengths and disrupt the hermetic seal needed to eliminate water transportation from the underlying dentin, the potential for “water-treeing” affected by osmosis is reduced. This breakthrough in technology renders an adhesive system that defies the typical label of the self-etching primer/adhesive “generation.”
In vitro clinical studies indicate that bond strengths exhibited by Bond-1 SFare as high as the results obtained when using traditional total-etch and self-etch adhesives, and restoration microleakage is comparable or superior when compared to four other leading competitive bonding agents.12,13 This self-etching adhesive comes in a traditional unit dose and a unique syringe delivery that permits the use of a flocked needle tip for easy, direct placement onto the preparation. By eliminating solvents from the formulation, evaporation during storage and prior to application is prevented, and longer working times provide a greater margin for error compared to similar products. The material is self-leveling and requires only one coat with no air-thinning prior to light-curing. Bond-1 SF Adhesive is indicated for use on direct composite restorations, and can be used with dual-cure materials when the initial layer of the dual-cure composite is light-cured.
Clinical Technique
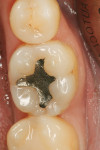
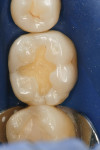
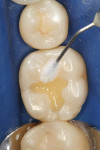
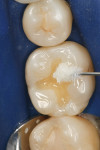
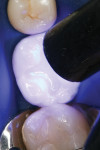
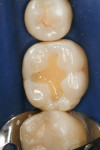
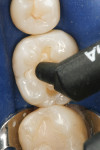
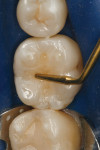
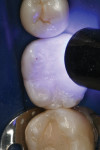
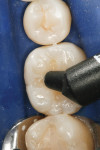
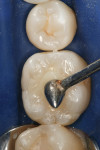
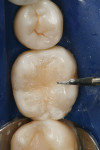
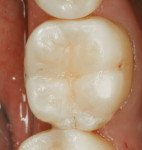
A patient presented with the desire to remove an unsightly amalgam restoration on tooth No. 19 (Figure 1). After anesthetization, rubber dam isolation was achieved and the amalgam was removed (Figure 2). For this case, the syringe delivery system of Bond-1 SF Adhesive was utilized (Figure 3). The screw cap was removed and the provided flocked tip was twisted onto the syringe (Figure 4). Because Bond-1 SF Adhesive is a self-etching system, no phosphoric-acid conditioning was necessary. Slight pressure was exerted on the plunger of the syringe to begin the flow of material through the flocked needle and applied to the dentin (Figure 5) and the enamel cavosurface (Figure 6) with a scrubbing motion for 20 seconds. The handling properties permit a smooth, even coat of material across the interface with a low film thickness without the need for air-thinning or volatilization of any solvents. Using a halogen light, Bond-1 SF was light-cured for 10 seconds (Figure 7). Figure 8 shows the cured layer of adhesive. A dentin body shade of composite (Artiste Shade A2 Dentin Body, Pentron Clinical Technologies) was applied and filled to the dentin-enamel junction in 2-mm increments without connecting the opposing lateral walls to manage polymerization stress on the bond (Figure 9). This layer was sculpted to replicate the cuspal slopes of the cavosurface and light-cured for 20 seconds (Figure 10 and Figure 11). A final layer of enamel composite (Artiste Shade A Enamel, Pentron Clinical Technologies) was applied and blended to the margins of the restoration with a ball burnisher to ensure full adaptation into the primary and secondary grooves (Figure 12). This layer was sculpted to match the existing cavosurface anatomy and light-cured for 20 seconds (Figure 13 and Figure 14). The rubber dam was removed and the occlusion was adjusted. The final result is shown in Figure 15.
Conclusion
As the technique and chemical formulations have advanced for the last 60 years, the classification of dental adhesives has been commonly segregated into “generations.” Beginning with simple phosphoric-acid conditioning of enamel and application of hydrophobic resin, each generation of adhesive offered progressive advances leading up to predictable, single-bottle, one-step formulations. The latest so-called “sixth-generation” materials are well-accepted for their ease of use and reduction in postoperative sensitivity. Until the release of Bond-1 SF Adhesive, other materials in this class have contained solvents that must be completely evaporated prior to light-curing. Bond-1 SF Adhesive is a solvent-free, self-etching formulation that removes the potential for inadequate evaporation of water and other traditional solvents, permitting a larger margin for error and reduction in the potential of transportation of dentinal fluid through the cured adhesive layer. While a traditional unit-dose delivery system is available, the flocked needle syringe provides a unique delivery system that is convenient and easy to use. Bond-1 SF Adhesive breaks the “generation gap” by offering a truly novel formulation that defies categorization as any “generation” and dramatically reduces chair time and the chance for postoperative sensitivity.
References
1. Hagger O. Neu Katalyysatoren zur polymerization der athene bei raumtemperatur (New catalyst for polymerization of ethylene at room temperature). Helv Chim Acta. 1948;31:1624-1631.
2. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849-853.
3. Buonocore M, Wileman W, Brudevold F. A report on a resin composition capable of bonding to human dentin surfaces. J Dent Res. 1956;35:846-851.
4. Bowen RL. Adhesive bonding of various materials to hard tooth tissues II. Bonding to dentin promoted by a surface-active co-monomer. J Dent Res. 1965;44:895-902.
5. American Dental Association Council on Dental Materials. Instruments and equipment. Dentin bonding systems: An update. J Am Dent Assoc. 1987;114:91-95.
6. Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Tokyo: Quintessence; 1998.
7. Kanca J. A method for bonding to tooth structure using phosphoric acid as a dentin-enamel conditioner. Quintessence Int. 1991; 22:285-290.
8. Gwinnett AJ, Tay FR, Wei SHY. Bridging the gap between overly dry and overwet bonding phenomenon of dentin hybridization and tubular seal. In: Shimono M, Maeda T, Suda H, Takaysashi K, eds. Dentin/Pulp Complex. Tokyo: Quintessence; 1996:359-363.
9. Yoshiyama M, Sano H, Carvalho RM, Pashley DH. Adhesive mechanisms of a self-etching/self priming adhesive resin to enamel and dentin. J Hard Tiss Biol. 1996;5:31-35.
10. Tay FR, Pashley DH, Suh BI, et al. Single-step adhesives are permeable membranes. J Dent. 2002;30:371-382.
11. Tay FR, Carvalho RM, Pashley DH. Water movement across bonded dentin—too much of a good thing. J Appl Oral Sci. 2004;12:443-469.
12. Data on file. Bond strength data by Jia W, Jin S, Turcotte J (Pentron Clinical Technologies, LLC, Wallingford, CT). Data available upon request.
13. Data on file. John Burgess, University of Alabama-Birmingham. Data available upon request.
About the Author
Jeff T. Blank, DMD
Private Practice
Carolina Smile Center
Fort Mill, South Carolina
Chief Clinical Instructor
New Millennium Education, LLC