Model-Based Guided Bone Expansion and Implant Placement
Clinical case using the model-based methodology to develop a restrictive surgical guide.
Lambert J. Stumpel, III, DDS
It has long been observed that once a tooth is removed from its bony housing, the resulting healing will create a volumetric reduction of the site.1-3 More recently, Araújo and Lindhe have suggested that the resorption of the buccal and lingual walls of the extraction site occurred in two overlapping phases.4 During the first phase, the bundle bone was resorbed and replaced with woven bone. Because the crest of the buccal bone wall was comprised solely of bundle, this modeling resulted in substantial vertical reduction of the buccal crest. The second phase included resorption that occurred from the outer surfaces of both bone walls. The reason for this additional bone loss is presently not understood.4 Sufficient volume of bone is a prerequisite for successful long-term dental implant osseointegration. The same research group is teaching that the mere placement of an implant in an extraction socket does not prevent this resorptive process from occurring.5 As the natural healing creates a given resorption, socket preservation techniques are aiming to alter this sequela of bone healing. Although the research is more recent in nature, it appears that the use of allografts are potentially useful in preserving some of the bone volume after extractions.6-8 With this in mind it might be easier in new extraction situations to “not lose it,” instead of needing to build back bone that has resorbed. Of course, the clinical situation will often present with a site that has indeed already healed with various levels of resorbtion. During the treatment planning phase, one will have to consider the need for the addition of bony volume. At that time, many different surgical options are available, all with their distinct pros and cons. Although all grafting procedures appear to be able to create implant success at a similar level compared to pristine sites,9 some of the more invasive procedures carry higher complication rates.10 Increased bone volume can be obtained by addition of bone and bone-like materials or the expansion of the local bone at the intended implant site.
Block grafting will require the harvesting of the patient’s or donor’s bone at various sites, such as the iliac crest, if large volumes are required; otherwise, intraoral sites like the chin, ramus, or tuberosity can be used.11-13 The use of a particulate xenograft eliminates the potential of donor site morbidity, and are often used with membranes to protect the graft site from epithelial in-growth.14,15 The use of tunneling techniques aspires to be less invasive by minimizing the length of the incisions.16,17 In larger sites, to prevent the collapse of the grafting material, titanium mesh can be employed to mechanically maintain the volume.18-21
If there is some availability of osteogenic medullar bone between the cortical plates, then expanding the site can be accomplished with various techniques. Distraction in both vertical and horizontal directions can create the desired bone volume.22-24 Ridge splitting uses some of the same principles, but without the use of dedicated distracters. Pioneered in the mid-1980s by Drs Scipioni, Bruschi, and Calesini, the technique does not require membranes or grafting material.25 A partial-thickness flap creates access to the bony ridge. A cut is made in the occlusal aspect of the crest and lateral-releasing bone cuts allow for the buccal displacement of the bone segment. The segment can be lateralized, while maintaining perfusion of the bone by the nature of the partial-thickness flap. The dental implant can most often be placed immediately, aiding and maintaining the lateralization.25-29 Many variations have been proposed, including different flap designs. It appears that the less invasive the approach, the better the bone will respond.30 Expanding the bone without releasing bone cuts was popularized by Summers.31 and, again, many variations have been described in the literature.32-34 Osteotomes of increasing diameter are used to gradually expand the bone. Benign paroxysmal vertigo, a disturbance of the equilibrium, as a result of the malleting, has been described as a possible side effect.35 The use of motorized expanders aims at expanding the bony site, without the need for percussion of the instruments. The osteotomes of increasing diameter have threats that will drive the instruments through the bone.36 Because of the increasing taper of the instruments, the bone is expanded gradually. The lingual cortex has been reported to be on average 30% thicker then the buccal cortex,37 so expansion will have a tendency to be more pronounced on the less resistive buccal compared to the lingual.
The motorized expanding procedure is started by an initial osteotomy with a small-diameter drill into the bony ridge. Conventionally, the site is exposed by means of a muco-periosteal flap; this will allow the clinician to evaluate the ridge and make the osteotomy with direct vision. As previously mentioned, exposure of the ridge may have a tendency to have a negative effect on bone volume, hence there is a potential for a flapless procedure to be advantageous as far as bone volume is concerned. The drawback of a flapless procedure is the elimination of direct visual feedback. Because of the already-compromised situation described here, this can only be accomplished dependably if some type of imaging, and with that, the development of a restrictive surgical guide, is utilized. Linear tomography and its digital cousins, computer tomography (CT) and cone beam CT (CBCT), are radiographic methods to understand the available bone volume, not visible with conventional two-dimensional radiographs. Computer-aided design (CAD) with computer-aided manufacturing (CAM) may be used to make a restrictive surgical guide that will guide the initial osteotomy into the predetermined position.38 Radiographic methods imply the exposure of the patient to ionizing radiation, and will always have to be governed by the ALARA principle: as low as reasonably achievable.39,40 The benefits of the imaging modality should be balanced against the ionizing radiation exposure and the possibility to acquire similar information in an alternative fashion.
Financial considerations will add to the decision-making process. Bone sounding is a non-radiographic alternative to develop an understanding of the available bone volume in the buccolingual dimension.41 The thickness of the mucosa is measured at multiple sites along the edentulous ridge. These measurements are transferred to a cast that has been cross-sectioned at the narrowest part of the ridge. This will now give a very accurate depiction of bone volume of the ridge part that has been captured in the impression.42 The area on the cast, below this part, should be considered carefully, as it holds no reliable diagnostic information. The author has previously described methods to develop a fully restrictive surgical guide, based on the model-based protocol.43
Following is a clinical case using the model-based methodology to develop a restrictive surgical guide. Because the protocol has been published previously, a condensed version will be described. The guide is used to create the initial osteotomy, guide the osteotomes, and finally guide the placement of the dental implant.
Clinical Case
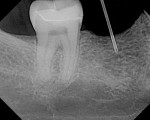
A 40-year-old healthy man presented with the desire to have an edentulous mandibular first molar site restored with the support of a dental implant. Clinical examination at site No. 30 revealed a moderately resorbed, healed area, with healthy periodontal support of the neighboring teeth. Evaluation of the local bony volume was carried out via palpation of the lingual cortex, and mylohyoid ridge; this was to appreciate the submandibular fossa. Periapical radiography showed adequate bone volume in the sagittal plane and the canal of the inferior alveolar nerve and mental foramen imaged clearly on the periapical radiographs. The combination of these examinations allowed the determination of the z-axis, which would determine the length and depth of the implant. A 10-mm long implant was selected, ensuring a safe distance from potentially dangerous areas, while allowing adequate osseo-integrative support. After application of topical anesthetic, a bone-measuring caliper was used to measure the buccolingual dimension. It was discovered that the crestal area measured 4 mm in width. Because this is inadequate for a regular-size implant body, the determination was made that an increase in volume in the buccolingual direction was needed. After discussion of the options, risks, and benefits, the patient consented to implant placement with simultaneous ridge expansion. An impression tray was extended around the proposed implant site with orthodontic tray wax, to improve the adaptation and maximize the captured crest height. A non-reversible hydrocolloid was used to capture the impression. The impression was then cast in a dedicated casting PVS material (Mach Slo/BluMousse, Parkell Inc, https://www.parkell.com) into a pin-less system (ACCU-TRAC® , Coltène-Whaledent, https://www.coltene.com). This would allow the cast to be sectioned; the individual pieces could then be repositioned back into the base of the system and would then again be correctly interrelated. A 0.75-mm thick acrylic-accepting vacuform template was formed onto the cast (Essix A+, DENTSPLY Raintree Essix, https://www.essix.com); this would be the foundation for the surgical guide to be produced.
To determine the mesio-distal plane, a staple was positioned into a stiff polyvinyl siloxane (PVS) putty preoperative impression in the estimated long axis of the proposed implant, a radiograph was taken, and the staple orientation was evaluated and modified as needed, to indicate the correct mesio-distal axis. The assembly would transfer the mesio-distal position to the cast. A short dental needle with an endodontic rubber stopper was used to measure the tissue thickness overlaying the crest. One occlusal, two buccal, and two lingual measurements were taken. The patient was dismissed and the laboratory phase was started.
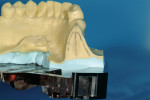
The putty-staple assembly was positioned onto the cast, and the long axis of the staple was transferred to the cast as this was the approved mesio-distal position of the axis of the proposed implant. The cast was removed from the base and was sectioned with a large diamond disk exactly according to the marked long axis of the staple. Once cut, the tissue-depth information that was previously recorded was now transcribed to the cast. The dots were connected and the soft tissue at the outside of the line could be marked. Consequently, the material inside of the line was the bone volume in a buccolingual direction (Figure 1). The desired implant position from a buccolingual direction was marked on the cast, guided by the available bone and the requirements for the prosthetic reconstruction. The axis was also marked on the crestal part of the cast, as this would guide the entry point of the drill bit during the cast osteotomy. At this time it was readily apparent that the crest width was insufficient (Figure 2).
The position of the top of the implant was marked on the cast and this horizontal position was extended laterally onto the cast. This depth was governed by available bone volume and the emergence profile requirements for the prosthesis. Most often this will range from 2 mm to 3 mm from the marginal gingival contour as proposed for the definitive restoration.
The mesio-distal plane was now represented by the surface created through the transverse cut cast, while the buccolingual axis had been transcribed onto the cast. First, the rotational component of these two planes had to be copied to a surveyor table. To do so, the Accu-trac tray was fitted with the part of the cast that had been marked with the disto-lingual axis. The tray members were closed so that the sectional cast was correctly related to the base of the tray. The assembly was placed into a surveyor table. A drill bit in the size corresponding with the analog size for the selected implant width was placed in a drill press. The surveyor table was positioned in the drill press. The press was lowered to place the drill bit at the level of the residual ridge on the sectioned cast. The surveyor table was unlocked and could now rotate freely in both x and y planes. The drill bit was placed flush with the transverse cut surface of the residual ridge to capture the mesio-distal plane and, while maintaining contact with said plane, the table was rotated to coincide with the marked axis of the buccolingual plane. The table was locked, and the angulations were rechecked. The surveyor table was now programmed with the rotation information for the x and y planes. The Accu-trac tray assembly was removed from the surveyor table; the remaining section of the cast was placed back into the tray, and the locking members were closed; this will have related the cast to its original configuration.
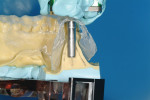
The tray assembly was now repositioned in the drill press. Because the rotational information for the model osteotomy was programmed in the surveyor table, the remaining translation component was set by moving the surveyor table under the tip of the drill bit, in the position that coincided with the marked crest and buccal cut. Once correct, the drill bit was lowered and a cast osteotomy was made, slightly deeper than the length of the analog combined with the soft tissue depth. All of the information needed to place the implant analog in the correct 3D position in the cast was now available. An implant analog 4.3 mm in diameter (Nobel Biocare, https://www.nobelbiocare.com), was secured with cyano-acrylate glue in the cast osteotomy (Figure 3). A metal guide sleeve with a 5-mm internal diameter is secured onto a guide cylinder with a pin (Nobel Biocare), and this assembly was connected onto the analog in the model. The previously made vacuform template was adjusted and the guide sleeve was connected to the vacuform shell with orthodontic acrylic resin. Upon setting, the surgical guide was finished.
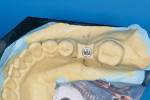
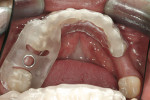
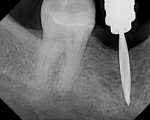
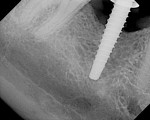
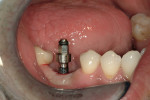
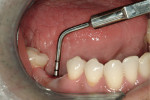
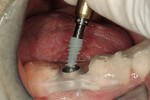
At the next clinical appointment the fitting of the surgical guide was confirmed (Figure 4 and Figure 5) and the site was anesthetized with buccal infiltration of half an ampule of articaine hydrochloride 4% with epinephrine 1:100,000. The remaining half an ampule was administered via an intraosseous delivery system(X-tip, Dentsply Tulsa, https://www.tulsadental.com). To preserve the keratinized gingiva, a crestal incision was made splitting the keratinized gingiva between the buccal and lingual site of the planned implant. The surgical guide was placed intraorally and a 2-mm surgical drill guide (Nobel Biocare), was used to guide the initial 2-mm twist drill to the predetermined depth for the planned 10-mm implant (Figure 9 and Figure 10). With a crest of 4 mm and an osteotomy of 2 mm, there was now 1 mm of bone on both the lingual and the buccal, which should be preserved. The motorized expanders come in a set of four with increasing diameter (motorized expander, BTI of North America, https://www.bti-implant.es). The top of the expander has a ring which corresponds to the 5-mm surgical sleeve in the surgical guide. The instruments are 20 mm in length from the tip of the instrument to the top of the ring. Because the surgical sleeve, per protocol, is positioned 9 mm above the top of the implant to be placed, if the top of the ring of the motorized expander and the surgical sleeve would be flush this would be consistent with an 11-mm osteotomy depth (20 minus 9).
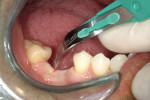
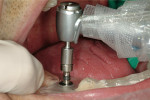
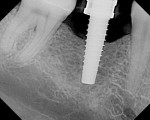
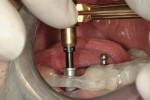
The second expander was introduced into the pilot hole, and initially visually guided as referenced to the surgical sleeve (Figure 11 and Figure 12). The ring on the instrument finally travelled through the surgical sleeve until it was 1-mm from being flush, as the osteotomy was intended for a 10-mm implant. This now expanded the site to 2.6 mm. The same procedure was repeated with the third expander (Figure 13 and Figure 14). As increasing resistance was felt of the bone, every half turn was followed by 30 seconds of inactivity to allow the bone to elastically expand. The third expander left the entrance of the osteotomy at 3.1 mm. The fourth and final expander was used and expanded to 3.6 mm (Figure 15 and Figure 16). The surgical guide was removed to confirm the integrity of the bony walls of the osteotomy (Figure 17 and Figure 18).
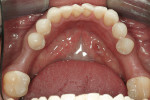
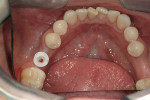
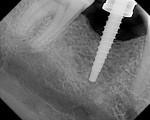
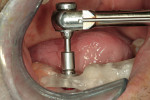
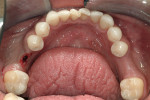
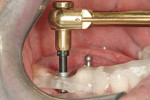
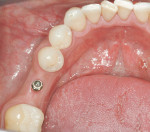
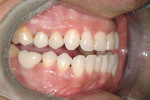
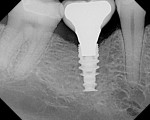
The implant being used was an actively drilling implant, with expanding capability (NobelActive). At the time there was not instrumentation to restrictively guide this implant through a surgical guide, so an implant driver from the system was modified (Figure 19) to allow the 3-mm wide shaft to be guided by a 3-mm drill guide. The implant size, 10 mm x 4.3 mm, was now introduced into the 3.6-mm wide osteotomy (Figure 20). The modified driver was placed into a 3-mm drill guide, which was placed into the surgical sleeve. As the implant was not yet seated, the relationship could be appreciated (Figure 21). The implant was gradually seated with the torque driver limiting the seating torque to 70 Ncm. This accomplished the final expansion of the ridge. A healing cap was placed and the patient was dismissed after receiving postoperative instructions. The patient reported minimal postoperative discomfort. Six weeks later, the patient presented for the prosthetic part of the treatment (Figure 23); the before and after images clearly show the increase in contour of the site. Standard prosthodontic procedures eventually allowed the delivery of a screw-retained crown (Figure 24 and Figure 25).
Conclusion
The bony volume of a deficient ridge will have to be augmented to allow predictable function of a dental implant. Many techniques are available to accomplish this. A method is described in this article for guided bone expansion and implant placement, allowing minimally invasive treatment for an otherwise complex situation.
References
1. Atwood DA. Some clinical factors related to rate of resorption of residual ridges. J Pros Dent. 1962;12:441-450.
2. Nakamoto RY. Bony defects on the crest of the residual alveolar ridge. J Prosthet Dent. 1968;19(2):111-118.
Pietrokovski J. The bony residual ridge in man. J Prosthet Dent. 1975;34(4):456-462.
3. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212-218.
4. Araújo MG, Sukekava F, Wennström JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32(6):645-652.
5. Araújo MG, Lindhe J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin Oral Implants Res. 2009;20(5):433-440.
6. Cardaropoli D, Cardaropoli G. Preservation of the postextraction alveolar ridge: a clinical and histologic study. Int J Periodontics Restorative Dent. 2008;28(5):469-477.
7. Cardaropoli G, Araújo M, Hayacibara R, et al. Healing of extraction sockets and surgically produced—augmented and non-augmented—defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32(5):435-440.
8. Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J Clin Periodontol. 2008;35(8 Suppl):173-202.
9. Uckan S, Veziroglu F, Dayangac E. Alveolar distraction osteogenesis versus autogenous onlay bone grafting for alveolar ridge augmentation: Technique, complications, and implant survival rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):511-515.
10. Pendarvis WT, Sandifer JB. Localized ridge augmentation using a block allograft with subsequent implant placement: a case series. Int J Periodontics Restorative Dent. 2008;28(5):509-515.
11. Nissan J, Romanos GE, Mardinger O, Chaushu G. Immediate nonfunctional loading of single-tooth implants in the anterior maxilla following augmentation with freeze-dried cancellous block allograft: a case series. Int J Oral Maxillofac Implants. 2008;23(4):709-716.
12. von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17(4):359-366.
13. Hämmerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19(1):19-25.
14. Barone A, Aldini NN, Fini M, et al. Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. J Periodontol. 2008;79(8):1370-1377.
15. Kfir E, Kfir V, Eliav E, Kaluski E. Minimally invasive guided bone regeneration. J Oral Implantol. 2007;33(4):205-210.
16. Hasson O. Augmentation of deficient lateral alveolar ridge using the subperiosteal tunneling dissection approach. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):e14-9.
17. Pieri F, Corinaldesi G, Fini M, et al. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2-year prospective study. J Periodontol. 2008;79(11):2093-2103.
18. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237-247.
19. Corinaldesi G, Pieri F, Marchetti C, et al. Histologic and histomorphometric evaluation of alveolar ridge augmentation using bone grafts and titanium micromesh in humans. J Periodontol. 2007;78(8):1477-1484.
20.Molly L, Quirynen M, Michiels K, van Steenberghe D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: a retrospective clinical assessment. Clin Oral Implants Res. 2006;17(5):481-487.
21. Funaki K, Takahashi T, Yamuchi K. Horizontal alveolar ridge augmentation using distraction osteogenesis: comparison with a bone-splitting method in a dog model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):350-358.
22. Lee HJ, Ahn MR, Sohn DS. Piezoelectric distraction osteogenesis in the atrophic maxillary anterior area: a case report. Implant Dent. 2007;16(3):227-234.
23. Laster Z, Rachmiel A, Jensen OT. Alveolar width distraction osteogenesis for early implant placement. J Oral Maxillofac Surg. 2005;63(12):1724-1730.
24. Scipioni A, Bruschi GB, Calesini G. The edentulous ridge expansion technique: a five-year study. Int J Periodontics Restorative Dent. 1994;14(5):451-459.
25. Koo S, Dibart S, Weber HP. Ridge-splitting technique with simultaneous implant placement. Compend Contin Educ Dent. 2008;29(2):106-110.
26. Enislidis G, Wittwer G, Ewers R. Preliminary report on a staged ridge splitting technique for implant placement in the mandible: a technical note. Int J Oral Maxillofac Implants. 2006;21(3):445-449.
27. Santagata M, Guariniello L, D’Andrea A, Tartaro G. A modified crestal ridge expansion technique for immediate placement of implants: a report of three cases. J Oral Implantol. 2008;34(6):319-324.
28. Bravi F, Bruschi GB, Ferrini F. A 10-year multicenter retrospective clinical study of 1715 implants placed with the edentulous ridge expansion technique. Int J Periodontics Restorative Dent. 2007;27(6):557-565.
29. Jensen OT, Cullum DR, Baer D. Marginal bone stability using 3 different flap approaches for alveolar split expansion for dental implants: a 1-year clinical study. J Oral Maxillofac Surg. 2009;67(9):1921-1930.
30. Summers RB. The osteotome technique: Part 2—The ridge expansion osteotomy (REO) procedure. Compendium. 1994;15(4):422-426.
31. Siddiqui AA, Sosovicka M. Lateral bone condensing and expansion for placement of endosseous dental implants: a new technique. J Oral Implantol. 2006;32(2):87-94.
32. Rambla-Ferrer J, Peñarrocha-Diago M, Guarinos-Carbó J. Analysis of the use of expansion osteotomes for the creation of implant beds. Technical contributions and review of the literature. Med Oral Patol Oral Cir Bucal. 2006;11(3):E267-E271.
33. Borgner RA, Kirkos LT, Gougaloff R, et al. Computerized tomography scan interpretation of a bone expansion technique. J Oral Implantol. 1999;25(2):102-108.
Peñarrocha-Diago M, Rambla-Ferrer J, Perez V, Pérez-Garrigues H. Benign paroxysmal vertigo secondary to placement of maxillary implants using the alveolar expansion technique with osteotomes: a study of 4 cases. Int J Oral Maxillofac Implants. 2008;23(1):129-132.
Lee EA, Anitua E. Atraumatic ridge expansion and implant site preparation with motorized bone expanders. Pract Proced Aesthet Dent. 2006;18(1):17-22.
Flanagan D. A comparison of facial and lingual cortical thicknesses in edentulous maxillary and mandibular sites measured on computerized tomograms. J Oral Implantol. 2008;34(5):256-258.
van Steenberghe D, Glauser R, Blombäck U, et al. A computed tomographic scan-derived customized surgical template and fixed prosthesis for flapless surgery and immediate loading of implants in fully edentulous maxillae: a prospective multicenter study. Clin Implant Dent Relat Res. 2005;7(Suppl 1):S111-S120.
Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100(24):13761-13766.
Wall BF, Kendall GM, Edwards AA, et al. What are the risks from medical X-rays and other low dose radiation? Br J Radiol. 2006;79(940):285-294.
Flanagan D. A nonradiologic method for estimating bone volume for dental implant placement in the completely edentulous arch. J Oral Implantol. 2001;27(3):115-117.
Perez LA, Brooks SL, Wang HL, Eber RM. Comparison of linear tomography and direct ridge mapping for the determination of edentulous ridge dimensions in human cadavers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):748-754.
Stumpel LJ 3rd. Cast-based guided implant placement: a novel technique. J Prosthet Dent. 2008;100(1):61-69.
About the Author
Lambert J. Stumpel, III, DDS
Private Practice
San Francisco, California