You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
After a tooth is removed from the jaw, bone will be lost in height and width.1 This resorption process will continue for the rest of a patient’s life unless either a bone graft or dental implant is placed.2 When there is bone loss in a person’s jaw, the negative consequences from a dental standpoint are numerous. These consequences include: the inability to place implants in the correct position to support the prosthesis; poor esthetic results from a final fixed or removable prosthesis; negative periodontal effects on adjacent teeth; and, eventually, negative structural changes in a person’s facial appearance.
Bone loss after a tooth is lost can also create negative anatomical changes that make it difficult for a patient to wear a removable tissue-borne prosthesis. The choices of materials that a clinician has to consider when grafting a ridge defect are numerous.Available grafting materials offer different handling properties, bone growth capabilities, and a wide variety of financial costs. These are topics that a clinician must consider when choosing a material to graft ridge defects.
The patient must also be informed about the products that are available so that decisions can be made with regard to philosophical apprehensions. If autogenous bone is going to be used, the patient should be made aware of the consequences of a second-site surgery with regard to postoperative healing and possible complications.
The advantages of DFDBA putty are numerous. The ease of use, lack of migration of graft material, osteoinductive properties, resistance to deformation and movement, and avoidance of a second site surgery are some reasons why a clinician might use this product as a graft choice.3
For successful grafting, certain clinical parameters must be practiced. The keys to grafting, as published by Misch, best summarize these principles.4 Use of an aseptic environment, obtaining soft tissue closure, and assessment of the defect size are some of the key principles of grafting bone with any material.
Review of Graft Material Choices
When a clinician decides to graft bone in a ridge defect, a choice of a graft material must be made. Each option offers different handling characteristics, different methods of preparation or attainment, and different properties within the graft site. A clinician should be ready to discuss these considerations with their patient as part of the treatment planning process.
There are four categories of graft materials in dental bone grafting: autogenous bone (same species and same genotype), allograft bone (same species and different genotype), xenograft bone (different species), and alloplast (synthetic product).5 When bone is grafted in a ridge defect, a graft material is remodeled over time. Remodeling is the process of the body turning over the graft material and forming bone. All grafting materials offer an osteoconductive matrix to a graft site, allowing the body to remodel the graft material into reformed bone. Xenograft and alloplast are only osteoconductive through the nature of their content.6
Osteoconductive materials are biocompatible, but will not grow bone without the presence of surrounding cells that will form bone. Any graft material that is used for a graft will act as a scaffold for bone growth to occur. Each material offers a different timeframe of resorption before new bone is formed, and each material offers different levels of the amount of resorption of material.7 For instance, xenograft resorbs relatively slowly because of a high amount of calcium in the product. This may be an advantage in some clinical situations, such as when a removable prosthesis is being supported.
Autogenous bone offers graft-site osteoinductivity, osteoconductivity, and is also osteogenic. Only a person’s own bone can be osteogenic, allowing the genesis of bone without the help of surrounding growth factors. Autogenous bone has the disadvantage of needing second-site surgery to obtain it.
While autogenous bone is also osteoinductive, only some allograft products are osteoinductive. Osteoinductivity is defined as new bone formation from the influence of inducing agents that come from the bone matrix. The available allografts for grafting can be demineralized freeze-dried bone or freeze-dried bone. According to an American Academy of Periodontology position paper in 2003, only DFDBA is osteoinductive.8 The additional step of demineralization exposes morphogenic bone proteins, allowing osteoinductivity. Allograft bone is harvested from cadavers and its safety is regulated by the FDA.9
Allograft bone is available in various handling forms, namely particulate form or putties. Various manufacturers recommend different hydration techniques for their particulate-form DFDBA products. Hydration can occur from saline or blood for most products. The time for hydration also varies between the manufacturers of the product. One disadvantage of any particulate form of graft material is the tendency toward migration from the graft site. This degree of migration can be somewhat controlled by the hydration technique.
Putty Forms of DFDBA
Putty-form DFDBA products can offer unique handling characteristics. The results of putty-form DFDBA products compare favorably to particulate DFDBA products in grafting results.10 The various putty-form DFDBA products that are available to the United States dental market at the time of writing this article are: Dynagraft™ and Orthoblast™ (IsoTis OrthoBiologics, Irvine, CA); Regenaform and Regenafil (Exactech, Gainesville, FL); Grafton® DBM (Osteotech, Eatontown, NJ); Puros® DBM Putty (Zimmer Dental, Carlsbad, CA); Regeneross, (Biomet 3i, Palm Beach Gardens, FL); and DBX Bone Matrix (Musculoskeletal Transplant Foundation, Edison, NJ). Many other DFDBA putty products are also available for the spine, podiatry, and orthopedic markets.11
The main advantage of a putty-form DFDBA is the ease of handling and lack of migration of material when placed into graft sites vs particulate DFDBA materials.12 The reduced migration of the graft material, especially in an aqueous or blood-laden environment, is a major advantage to the dental clinician. This lack of migration of grafting material becomes important in larger ridge defect cases. Various carriers in the DFDBA putty offer handling properties unique to each product. Some of the carriers used in DFDBA putty products are glycerin, porcine gelatin, calcium stearate, lecithin, sodium hyaluronate, polymers, and collagen matrixes.Various proprietary reverse-phase mediums allow for a hardening of the material at body temperature. The carrier matrix has the potential to allow for vascular in-growth because of its space-producing effect.13 This in-growth is a key to a successful graft.
When DFDBA putties are thermoplastic in form, it allows for precise molding of the material into the graft site and shaping of the graft form. All DFDBA putty products offer various degrees of shape memory. This ability to shape the graft and maintain memory is a major advantage of DFDBA putty materials. When a graft product is in a putty form, the author has found it easier to manipulate the product in the graft site vs particulate materials. The clinician can use a glove wetted with sterile saline to mold the putty material where needed.
Another clinical advantage to DFDBA putty is its hemostatic effect on a graft site. The absorbent nature of the carrier and contact with the site allows for reduction of bleeding.
If cortical bone is incorporated into DFDBA putty, then the benefit of radiopacity is offered to the product. Cortical chips also create a denser product for handling purposes and increased mineralization.14 This is also an advantage when a radiograph is taken for a postsurgical placement check.
Treatment Planning for Grafting
A review of a patient’s medical history is the start for treatment planning a grafting case. Any uncontrolled disease, pregnancy, or uncontrolled drug use or alcohol intake are the absolute contraindications to a graft being performed on a patient. Smoking should be deterred but is only a consideration with regard to grafting.
Understanding the drugs that a patient is currently taking, allergies, and previous notable medical problems are important to the treatment planning process. Dialogue with the patient in reference to treatment options and alternatives is another important component to treatment planning for a graft. A discussion of which materials will be used for the graft and its safety is also an important step. It is the author’s opinion that a cone beam computed tomography (CBCT) scan should be obtained before grafting. A CBCT scan offers unparalleled visual information that allows a surgeon to plan correctly for volume of graft material, visualization of anatomical parameters, and coordination of the prosthetic end result in relation to the graft.15
An interactive CBCT scan offers a 3D view that allows visualization of a ridge defect with lifelike reality. In the maxillary arch, a CBCT scan shows the anatomy of the maxillary sinus with regard to pathology and available bone for dental implant placement. In the mandible, the inferior alveolar nerve can be traced and measured. A CBCT scan also shows the density of surrounding bone before grafting. This can indicate a need for grafting for improved mineralization and volume. A CBCT scan also offers the advantage of reduced radiation exposure over a fan-beam traditional CT scan.
Other advantages of a CBCT scan are the in-office availability, expedient results, and interactive format. Through using one of the many interactive CBCT programs available, bone density, volume measurements, virtual implant placement, and 3D volumetric visualization can be obtained.16
Another important aspect of treatment planning with respect to ridge augmentation is consideration of provisionalization during healing of the graft. An ideal provisional restoration will provide esthetics and function during healing, yet will avoid movement of the graft material. In general, the more stable a provisional restoration, the better it is for graft healing.
Healing time for a grafted site is normally between 4 to 6 months. This is dependent on defect size and the healing capacity of the patient. A fixed provisional bridge is preferred to a removable appliance as it offers increased stability during healing. A removable denture often requires relining with a soft reline material during the healing process. When tooth preparations are contraindicated, bonding is an acceptable alternative to a bridge. One advantage of DFDBA putty materials is their resistance to movement from an overlying provisional. The firmness of the thermoplastic DFDBA putty product is especially resistant to deformation from provisional pressures.
Principles of Grafting
To successfully graft a bone defect, published principles of grafting should be followed. The keys to grafting, as published by Misch, outline these principles in a clear manner.4 Some of these keys are:
Asepsis: When remodeling a graft material, bacterial infection could create an osteolytic environment, which can cause poor graft results. The lower pH that an infection causes is counterproductive to a successful graft. The removal of granulation tissue in the graft site is the first step toward a successful graft. Through this removal of tissue, the site is offered an initial debridement. By following an aseptic set-up during the grafting procedure, the surgeon can avoid exogenous bacteria from entering a graft site.
Another way to avoid infection from being a detriment to a graft is to not graft in the presence of active infection. If puss is evident in a defect, it is better to place the patient on antibiotics and graft at a later time. Antibiotics also negate endogenous bacteria that could be a detriment to a bone graft.
Soft Tissue Closure: Through obtaining closure of soft tissue over a graft site, the graft is better maintained, has reduced invasion of debris, and has better vascularity. Various tissue-releasing techniques are available that allow proper flap closure. One technique includes carefully scoring the periosteum and stretching the flap.17 Sometimes connective tissue grafts can be added during a hard tissue graft to improve keratinization of the surrounding tissue and assist with closure. Proper suturing techniques also assist in maintaining closure. The key is to have the tissue cover the graft in a flaccid manner before suturing, thus allowing the sutures to gently keep closure. With putty DFDBA materials, it is easy to overbulk the graft site because of its rigidity. This overbulking can make closure more difficult and technique-sensitive.
Defect Size/Topography: Having an understanding of the defect size being grafted is an important concept to successful grafting. The more walls of bone that a graft site offers, the more predictable the graft will be.18 The remaining walls of bone offer containment of the graft and increased vascularity to nourish the graft.When the buccal or lingual wall of bone is missing, then a membrane should be used to contain the graft material and exclude soft tissue invasion.
Thermoplastic DFDBA putty graft materials offer a tenting effect that helps to protect the graft from movement and supports the membrane. Collagen membranes offer the advantage of protecting the graft and a second surgery is not required to remove it. Another advantage of DFDBA putties is their ability to mold into the defect with ease. This can be attained with a glove wetted with saline. This is in contrast to many particulate materials that tend to migrate from the site, particularly when placed in an aqueous environment.
Case Example 1
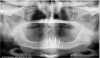
A 56-year-old woman in good health presented with recent extractions and a fully edentulous maxillary arch and edentulous posterior mandibular areas. A CBCT scan 3D view revealed large osseous defects in the maxillary arch that required grafting before implant placement (Figure 1). The treatment plan was made for maxillary ridge augmentation followed by fixed prosthesis-supported dental implants in all of the edentulous areas. The mandible did not require grafting. The patient was wearing a full upper denture during the entire grafting and subsequent implant healing time period. The maxillary arch showed large defects on the axial view of the CBCT (Figure 2). A cross-sectional CBCT view also showed deficient height of bone with a vertical height of 9.7 mm (Figure 3). Upon full-thickness reflection, large deficient areas of the ridge were seen that required grafting before implant placement (Figure 4).
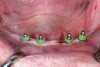
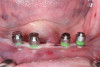
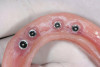
A DFDBA bone-graft putty (Regeneform) was used to graft the entire maxillary arch. All extraction defects were degranulated, the bone was decorticated to allow bleeding, then the DFDBA putty was placed and covered with resorbable collagen membranes (Biomend Extend, Zimmer Dental) (Figure 5). Primary closure was obtained and the patient wore her denture after a soft reline for a period of 6 months (Figure 6). At 6 months post-graft, a CBCT scan revealed substantial graft material from both an axial (Figure 7) and a crosssectional view, showing more than 15 mm of bone height (Figure 8). The 3D view from the CBCT also showed substantial ridge changes in both height and width (Figure 9). Using a CBCT software program (SimPlant, Materialise Dental Inc, Glen Burnie, MD), implants were planned for placement (Figure 10). As per the CBCT plan, 11 internal hex implants (BioHorizons, Birmingham, AL) were placed in the grafted bone (Figure 11). The ideal bone height allowed implant placement in an ideal position with relation to esthetics and occlusion.
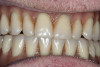
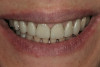
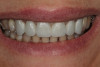
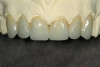
Through grafting, an ideal crown-to-implant ratio was obtained with relation to the final prosthetic position. A panograph reveals implant placement (Figure 12). Esthetically, the patient ended up with a fixed implant-supported prosthesis that approximated her natural tooth position (Figure 13).
Case Example 2
A 53-year-old woman in good health presented for the replacement of missing teeth on the maxillary arch and periodontal therapy of the mandibular arch. A CBCT scan was taken that revealed the need for bone grafting on the maxillary ridge and maxillary sinus areas in the panographic (Figure 14) and axial (Figure 15) views. Bone loss was noted on the facial plate areas of the anterior ridge. A treatment plan was created to graft the maxillary ridge and sinuses bilaterally, and then to place 12 dental implants to support a fixed prosthesis on the maxillary arch.The treatment plan for the mandible consisted of periodontal surgery to save her remaining teeth.
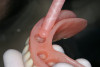
Upon full-thickness reflection of the maxillary ridge, defects that extended to the facial aspect were observed (Figure 16). After degranulation and perforation for bleeding of the extraction sites and defects, DFDBA putty (Regeneform) was placed into the defects and then covered with a resorbable collagen membrane (Ossix Plus, Colbar Lifescience, Rehovot, Israel) (Figure 17). The maxillary sinuses were grafted using a combination of DFDBA particulate (Puros) and xenograft particulate (Bio Oss®, Osteohealth, Shirley, NY) in a 50/50 combination. A lateral approach was used to graft the sinuses bilaterally. Both the ridge defects and sinuses were grafted simultaneously.
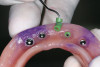
After 6 months of healing, a CBCT scan was taken and good bone formation was seen on the axial (Figure 18) and panograph (Figure 19) views. The CBCT was then used to plan for placement of dental implants (Figure 20). Upon full-thickness reflection of the ridge, abundant bone contour was observed. Internal hex dental implants (BioHorizons) were placed according to the locations planned on the CBCT (Figure 21). After a healing period of 4 months, the implants were uncovered (Figure 22) and abutments were placed (Figure 23). A panograph taken 1 year after this stage revealed the final restorations on well-integrated implants (Figure 24). The patient’s smile reveals a fixed prosthesis that simulates her natural teeth (Figure 25).
Conclusion
Grafting bone to restore defects in an atrophic ridge can offer many benefits to a patient. Through grafting, osseous support for dental implants can be achieved, periodontal support for adjacent teeth can be created, and soft tissue support for pontic areas and esthetic improvement occurs. There are many choices of grafting materials available to the dentist. The benefits of using DFDBA putty include ease of placement, reduced migration from the graft site––even in an aqueous environment—rigidity for membrane support, and osteoinductive properties. These handling properties become more important when large defect areas are being grafted. In larger graft sites, large quantities of graft material can be obtained without the need for a second-site surgery. When the principles of grafting are followed, large defects can be successfully grafted with DFDBA putty. The cases presented in this article show clinical success supported by 3D CBCT radiography.
Disclosure
The author has received honoraria from and is a current consultant for BioHorizons Inc. He is also the director of implant education for Microdental Laboratories.
References
1. Carlsson GE, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures: a longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy. 1967;18:27-54.
2. Christensen GJ. Ridge preservation: why not? J Am Dent Assoc. 1996;127:669-670.
3. Babbush CA. Histologic evaluation of human biopsies after dental augmentation with a demineralized bone matrix putty. Implant Dentistry. 2003;12(4):325-331.
4. Misch CE. Contemporary Implant Dentistry. 2nd ed. St Louis, Mo: Mosby; 1999:451-463.
5. Rose LF, Rosenberg E. Bone grafts and growth and differentiation factors for regenerative therapy: a review. Pract Proced Aesthet Dent.
2001;13(9):725-734.
6. Taylor JC, Cuff SE, Leger JP, et al. In vitro osteoclast resorption of bone substitute biomaterials used for implant site augmentation:a pilot study. Int J Oral Maxillofac Implants. 2002;17(3):321-330.
7. Karabuda C, Ozdemir O, et al. Histological and clinical evaluation of 3 different grafting materials for sinus lifting procedure based on 8 cases. J Periodontol. 2001; 72(10):1436-1442.
8. Committee on Research, Science and Therapy of the American Academy of Periodontology. Tissue banking of bone allografts used in
periodontal regeneration [position paper]. J Periodontol. 2001;72: 834-838.
9. Troyer D. Biorepository standards and protocols for collecting, processing, and storing human tissues. Methods Mol Biol. 2008; 441:193-220.
10. Bender SA, Rogalski JB, et al. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol. 2005;76(5):768-777.
11. Peterson B, Whang PG, Iglesias R, et al. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A(10):2243-2250.
12. Callan DP, Salkeld SL, Scarborough N. Histologic analysis of implant sites after grafting with demineralized bone matrix putty and sheets. Implant Dent. 2000;9(1): 36-44.
13. Wironen JF, et al. Effect of bone protein and carrier matrices on BMP-stimulated osteogenesis. Transactions of the 44th Annual
Meeting. Orthopaedic Research Society, 1998;23(2):603.
14. Boyan BD, Ranly DM, McMillan J, et al. Osteoinductive ability of human allograft formulations. J Periodontol. 2006;77(9): 1555-1563.
15. Tischler M. In-office cone beam computerized tomography: technology review and clinical examples. Dent Today. 2008;27(6): 102-106.
16. Tischler M. Interactive computerized tomography for dental implants. Treatment planning from the prosthetic end result. Dent Today. 2004;23(3):90-93.
17. Fugazzotto PA. Maintaining primary closure after guided bone regeneration procedures: introduction of a new flap design and
preliminary results. J Periodontol. 2006; 77(8):1452-1457.
18. Elian N, Cho SC, Froum S, Smith RB, Tarnow DP A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;19(2):99-106.
About the Author
Michael Tischler, DDS
Private Practice
Woodstock, NY