You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The first time a patient is examined, it is difficult to determine if the rate of wear is excessive. The only way to tell is if the patient has been a patient of record for a number of years. What constitutes “normal” wear rates? Various published articles have conflicting values. One study reports a normal loss of enamel between 20 µm and 38 µm per year.1 Another study reports a wear of 65 µm in 6 months.2 Noncarious tooth wear is a normal physiologic process that occurs in many patients throughout life.3 If the rate of wear is such that it is of concern to the patient, or if it is likely to prejudice the survival of the teeth, then the rate is considered to be pathologic, and action must be taken to minimize the damage.4,5
Erosion is defined as “the progressive loss of tooth substance by chemical processes that do not involve bacterial action.”6 Acid reflux appears as one of the most common causes of dental erosion. Erosion can be divided into extrinsic and intrinsic factors. The extrinsic factors include acid from beverages and foods, vigorous toothbrushing, and some oral medications. Intrinsic erosion is often caused by vomiting, gastric reflux, pregnancy, quality and quantity of saliva, and alcoholism.7 Because saliva rinses away and buffers acids on tooth surfaces, low salivary flow rates may be an initiating factor in dental erosion. It has also been reported that anorexics and bulimics develop xerostomia and their saliva may have a lower buffering and remineralizing capacity.8
Most individuals experience gastroesophageal reflux at some time in their lives. GERD, however, is a clinical condition that occurs when the reflux of stomach acid into the esophagus is severe enough to impact the patient’s life and/ or damage the esophagus.9 A relationship between GERD and dental erosion has been described in a number of publications.10-14
In most patients, GERD results from a transient relaxation of the sphincter that keeps the lower end of the esophagus closed when he or she is not swallowing food or liquids, which allows acid and food particles to reflux into the esophagus.9 GERD is characterized by the chronic, intermittent, unrestricted movement of stomach acids into the esophagus. This is defined as regurgitation and should be distinguished from vomiting as it involves a passive or effortless return of the stomach contents into the mouth versus a physiologic response to stimuli controlled by the autonomic nervous system.12-14 The four major symptoms of GERD are: heartburn (uncomfortable, rising, burning sensation behind the breast bone), epigastric and retrosternal (noncardiac) pain, regurgitation of gastric acid or “sour stomach” contents into the mouth, and difficult and/or painful swallowing.12-14 GERD can be effectively managed with medication or lifestyle changes. Surgery is an option in severe cases. The frequent regurgitation of stomach acids into the mouth results in continuous undesired contact of these acids with the teeth in the oral cavity. This can lead to dental erosion. Dentists need to familiarize themselves with the consequences of GERD and know how to treat it. Erosion is different from abrasion in cause and appearance. As stated previously, erosion is a nonbacterial, chemical dissolution of hard tooth surfaces, whereas abrasion is caused by mechanical wear of tooth structure by external agents. The appearance of the lesions is different in that tooth surfaces affected by erosion have a spoon-shaped appearance, while abrasive lesions appear sharp, flat, and angular. Moreover, because erosion does not affect metal or plastic dental restorations, these remain as prominent elevated plateaus.15
Dental erosion can be the result of various systemic conditions, which often makes the etiology difficult to identify. These conditions include upper gastrointestinal disorders with an acid diet (43%), upper gastrointestinal disorders without an acid diet (25%), an acid diet (24%), eating disorders (6%), and unknown causes (2%).15 Unfortunately, the cause of dental erosion often goes undiagnosed, or the presence of other factors such as abrasion and attrition make diagnosis more difficult to determine.16
Bargen and Austin17 were the first to identify and report a relationship between dental erosion and gastrointestinal disturbances in a case report of a woman who presented with chronic vomiting. The erosion was primarily evident on the palatal surfaces of the anterior maxillary teeth. Eccles and Jenkins found a relationship between erosion of the lingual surfaces of anterior teeth and GERD.18,19 They suggested the following grading system for erosion: grade I is loss of enamel surface texture with no dentin involvement; grade II is erosion involving dentin for less than one third of the areas of the tooth surface; and grade III is dentin erosion involving more than one third of the tooth surface. Bartlett and colleagues reported that there was a strong relationship between palatal dental erosion and GERD, even in those patients with no symptoms of reflux.12-14
The damage caused to the dentition by GERD depends on the severity of the case. In the majority of cases, the occurrence of pathological reflux was noted to occur during the day and these findings are consistent with many other studies. However, if regurgitation of the gastric juice occurs at nighttime, when salivary flow is at its lowest, the potential for damage to the teeth increases significantly.20
Several authors have reported a high incidence of erosion among patients with psychological and psychiatric disorders and patients taking certain medications such as tranquilizers or beta-blocking agents.9 These medications produce a reduction in salivary secretion rates that contributes to dental erosion.21 While other investigators were unable to determine a significant relationship between the use of anticonvulsant drugs and dental erosion,22 evidence suggested that this type of medication decreased the pressure of the lower esophageal sphincter, making such individuals more likely to suffer from reflux.21,22
It has not been determined if the quality of oral hygiene has an effect on the severity of dental erosion.22 It has been reported, however, that mechanical factors such as occlusal wear, abrasive tongue action, and toothbrushing can potentiate the destructive nature of the acids. Ideally, the restoration of eroded tooth structure should occur following appropriate diagnosis and control of the etiology, and it should be oriented toward the re-establishment of function and esthetics. Other researchers emphasize the importance of early intervention before the progressive erosion makes it an almost-impossible task or a full-mouth rehabilitation becomes necessary.22
To efficiently treat tooth erosion, a comprehensive understanding of the effects of tooth wear must be obtained. Potential results of tooth wear can be related to functional, esthetic, and sensitivity concerns.23
Functional: Patients tend to have difficulty with mastication, broken teeth, and failing restorations.
Esthetic: As the dentition undergoes wear and fracture, the patient’s general appearance may change as he or she reveals fewer teeth during speech and natural smile. In some instances, the smile line may be reversed entirely.
Sensitivity: While most patients with excessive tooth wear often experience reduced sensitivity as a result of exposed dentin, some patients have reported preoperative hypersensitivity.
Minimally invasive treatments are procedures that restore form, function, and esthetics with minimal removal of sound tooth structure.24-26 As a person ages, so do their teeth and previously placed restorations. Eventually, teeth that have been restored will break down and patients will need to have those restorations replaced.27,28 Fortunately, restorative materials and procedures are constantly evolving. A conservative initial restoration will retain more tooth structure to work with at a second restoration.
Case Presentation
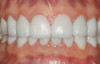
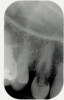
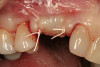
A 55-year-old patient was referred to the office with a severe (grade III) generalized loss of enamel and dentin. The patient was aware that his teeth were becoming thinner and was concerned that some of the anterior teeth might fracture (Figure 1). The patient was not aware of any systemic problems and did not have severe symptoms of acid reflux. Before proceeding with dental treatment, the patient was referred to a gastroenterologist for a full diagnostic exam. After the diagnostic exam, which included the monitoring of acid regurgitation and pH while the patient slept, a final diagnosis of GERD was rendered. The patient was treated with a gastric secretion suppressor medication (Prilosec®, Procter & Gamble, Cincinnati, OH) that inhibits the hydrogen/potassium ATP-ase enzyme system in the gastric parietal cells of the lining of the stomach. It is considered a gastric acid pump inhibitor because it works by blocking the final step of acid production.
Clinical Exam
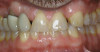
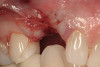
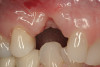
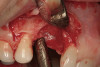
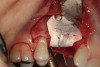
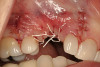
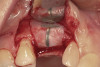
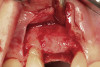
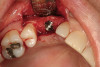
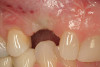
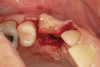
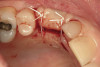
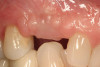
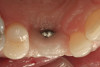
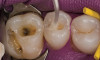
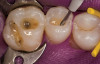
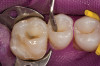
No abnormalities, asymmetry, or temporomandibular dysfunction were evident on extraoral examination. Intraoral examination revealed deep erosive lesions with a severe lack of enamel on all functional cusps and the cervical areas of several teeth. Severe wear of the maxillary incisal edges was observed and excessive “cupping” was noted on the palatal aspects of the maxillary anterior teeth (Figure 2 through Figure 6). Esthetic evaluation revealed a normal smile line with a previously restored diastema that was discolored. There was no evidence of periodontal disease, and the patient’s oral hygiene was satisfactory.
The pulps of numerous teeth were nearly exposed, particularly in the anterior region. Occlusal analysis revealed a Class I molar relationship. There was minimal to no overjet and almost 100% overbite. Because of the severe wear of the teeth, it was possible that the vertical dimension of occlusion was lost, but it would be only possible to confirm this if the patient had cephalometric x-rays from years ago that could be compared with their current appearance.
Treatment Plan
This case could have been treated a number of different ways. Orthodontics was an option given to the patient to allow room for restorative material in the anterior region. This would be the most conservative treatment option as it would allow the anterior teeth to be restored and the posterior teeth to be conservatively restored without changing the vertical dimension. The teeth would be moved to the anterior direction and intruded to allow room for restoration. However, the patient did not want to proceed with orthodontics.
Because the anterior teeth were very thin with severe notching and there was not enough overjet to restore function, they would be severely compromised and the risk of fracture would be high if they were prepared for full-coverage crowns. However, the posterior teeth could be treatment planned for partial-coverage restorations fabricated from gold, porcelain, or composite. Because orthodontics was not an option, the vertical dimension of occlusion needed to be altered to restore the patient’s teeth properly. Considering the findings and the patient’s concern of possible fracturing of the anterior teeth, a minimally invasive treatment plan was presented that would consist of fabricating lingual veneers made indirectly out of composite resin and composite onlays for the posterior mandibular molars. These restorations would be fabricated by the dentist from a diagnostic wax-up using a silicone die material (eg, quick-die silicone material, Bisco, Schaumburg, IL, or Mach-2®, Parkell, Inc, Edgewood, NY) and a clear polyvinyl siloxane (PVS) material (eg, RSVP, Cosmedent, Chicago, IL).
Diagnostic impressions were made out of a polyether material (Impregum™, 3M ESPE, St. Paul, MN) so that the silicone die material would not adhere to it when the models were fabricated. If a PVS material is used, the silicone die material will adhere to the impression and cannot be removed. The models were sent to the laboratory so that a Kois deprogrammer could be fabricated to facilitate mounting the case in centric relation. The patient wore the appliance for 3 weeks and then bite registration impressions were made with the appliance in place to capture the jaw position. Once the casts were mounted on an articulator, wax was added to the lingual of the anteriors (Figure 7) to restore the tooth structure that was missing. By adding the wax to the lingual of the anteriors, this would open the articulator so that the posteriors could be waxed (Figure 8) to the new vertical dimension. The minimal amount of opening was achieved so that the teeth could be restored properly.
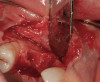
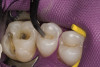
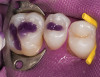
Once the wax-up was completed, PVS impressions were made to set up a matrix to form the new restorations in the posterior. The polyether impressions were injected with the silicone die material (Figure 9) and allowed to set for 2 minutes. The silicone die material was then removed from the impression and hybrid composite was added to form the lingual veneers (Figure 10). This was done freehand and only enough material to replace what was missing was added. This is how the new vertical dimension was determined. A clear polyvinyl matrix also could have been used for fabricating these restorations. The composite was light-cured and then placed in a Triad® unit (DENTSPLY Trubyte, York, PA) for final light-curing (Figure 11), which should make the restorations stronger. The restorations were finished and polished using finishing disks and polishing points (Figure 12) after first being micro-etched with 50-µm aluminum oxide powder, then etched with hydrofluoric acid (Figure 13) for 90 seconds, and finally silanated. The restorations were adhesively bonded with resin cement and then tried in the mouth for fit (Figure 14), before being cleaned in an ultrasonic bath with acetone. The teeth were isolated with an Expandex retractor (Parkell) and micro-etched using aluminum oxide powder (Figure 15) for 20 seconds (Figure 16) with Ultra-Etch (Ultradent, South Jordan, UT), then rinsed and air-dried. A fourth-generation bonding system (All-Bond 2®, Bisco) was used for its excellent bond strength. The two-part primer was mixed in a dispensing well and applied in numerous coats to the moist surface (Figure 17). This was followed by an application of the unfilled D/E resin (Bisco). The restoration was treated with D/E resin and Insure resin cement (Cosmedent). The adjacent teeth were protected from the etchant and adhesive by using Teflon® tape (E. I. du Pont de Nemours and Company, Wilmington, DE). The restorations were seated one at a time and light-cured for 40 seconds on the lingual and facial aspects. Figure 18 shows the first three restorations placed and the same procedure was followed for the remaining anterior restorations.
To fabricate the posterior restorations, a clear silicone impression tray— fabricated from the diagnostic wax-up—was used (Figure 19). The tray would be filled with composite resin and placed over the silicone die material. To make the composite resin more flowable, a Calset™ composite heater (AdDent, Danbury, CT) was used. The composite was injected into the clear matrix and placed on the silicone die material (Figure 20 and Figure 21). The clear impression tray was seated over the silicone die material and light-cured for 40 seconds. The tray was removed and the restorations are shown on the silicone die (Figure 22). These restorations were then separated, finished, polished, and were ready to be bonded on the posterior teeth.
Rubber dam isolation was used in the posterior for ideal moisture control. The teeth were etched with a microetcher first (Figure 23) and then etched with phosphoric acid. The same bonding protocol was used as the anterior restorations. To keep the etch from the adjacent teeth when bonding, a Brasseler serrated saw blade (Brasseler USA, Savannah, GA) was inserted interproximally because the contacts were not broken (Figure 24). A clear matrix would not go through the contacts. The restorations were then bonded individually (Figure 25) and the resin cement cleaned up. Figure 26 shows the lower right quadrant bonded to place before removing the rubber dam.
Once all of the posterior restorations were bonded to place, final equilibration was necessary. A composite platform was fabricated and placed on the lingual side of the upper anterior teeth, similar to using a Kois deprogrammer (Figure 27). The platform was retained mechanically by the diastema without any adhesive. The patient lightly occluded on the platform for about 15 minutes to allow the muscles to relax. The platform was then slightly modified (Figure 28) to allow the first tooth to touch. This would represent the initial contact in centric relation. Equilibration was then carried out until even, simultaneous contacts were present on all the posterior teeth and cuspids. The final equilibration, to remove any interference on the lingual of the anteriors when chewing, was accomplished with the patient chewing gum in the posterior zone while placing 200 µm articulating paper in the anterior zone. Only streaks and aberrant lines needed to be removed as these illustrate areas of friction within the envelope of function.
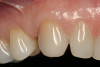
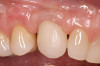
The patient elected to have the diastema closed and all the cervical lesions were restored with direct composite resin (Renamel® Microfill, Cosmedent). The final results are shown in Figure 29 through Figure 34.
Conclusion
The treatment of this patient could have been planned in several ways, finally opting for a procedure with no tooth removal. The conservative nature of the treatment will allow future restorations to be accomplished without worrying about destruction resulting from previous treatment. The restorations are easily repaired and should provide years of service.
REFERENCES
1. Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res. 1989;68(12): 1752-1754.
2. Xhonga FA. Bruxism and its effect on the teeth. J Oral Rehabil. 1977;4(1):65-76.
3. Flint S, Scully C. Orofacial age changes and related disease. Dent Update. 1988;15(8): 337-342.
4. Smith BG, Knight JK. An index for measuring the wear of teeth. Br Dent J. 1984; 156(12):435-438.
5. Watson IB, Tulloch EN. Clinical assessment of cases of tooth surface loss. Br Dent J. 1985;159(5):144-148.
6. The Academy of Prosthodontics. The glossary of prosthodontic terms. J Prosthet Dent. 1994;71(1):41-112.
7. Levitch LC, Bader JD, Shugars DA, Heymann HO, Non-carious cervical lesions. J Dent. 1994;22(4):195-207.
8. Hurst PS, Lacey LH, Crisp AH. Teeth, vomiting and diet: A study of the dental characteristics of seventeen anorexia nervosa patients. Postgrad Med J. 1977;53(620): 298-305.
9. Ibarra G, Senna G, Cobb D, Denehy G. Restoration of enamel and dentin erosion due to gastroesophageal reflux disease: a case report. Pract Proced Aesthet Dent. 2001;13(4): 297-304.
10. Jarvinen V, Meurman JH, Hyvarinen H, et al. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol. 1988;65(3):298-303.
11. Taylor G, Taylor S, Abrams R, Mueller W. Dental erosion associated with asymptomatic gastroesophageal reflux. ASDC J Dent Child. 1992;59(3):182-185.
12. Bartlett DW, Evans DF, Smith BG. The relationship between GERD disease and dental erosion: Rev J Oral Rehabil. 1996;23(5): 289-297.
13. Bartlett DW, Evans DF, Anggiansah A, Smith BG. A study of the association between GERD and palatal dental erosion. Br Dent J. 1996;181(4):125-132.
14. Bartlett DW, Smith BGN. Clinical investigations of GERD: Part1. Dent Update. 1996; 23(5):205-208.
15. Bouquot JE, Seime RJ. Bulimia nervosa: a dental perspective. Pract Periodontics Aesthet Dent. 1997;9(6):655-663.
16. Cardoso AC, Canabarro S, Myers SL. Dental erosion: Diagnostic-based noninvasive treatment. Pract Periodontics Aesthet Dent. 2000;12(2):223-228.
17. Bargen JA, Austin LT. Decalcification of teeth as a result of constipation with long-continued vomiting: report of a case. J Am Dent Assoc. 1937;24:1271.
18. Eccles JD, Jenkins WG. Dental erosion and diet. J Dent. 1974;2(4):153-159.
19. Eccles JD. Tooth surface loss from abrasion, attrition, and erosion. Dent Update. 1982;9(7):373-381.
20. Smith BG, Robb ND. Dental erosion in patients with chronic alcoholism. J Dent. 1989;17(5):219-221.
21. Meurman JH, Toskala J, Nuutinen P, Klemetti E. Oral and dental manifestations in GERD. Oral Surg Oral Med Oral Pathol. 1994; 78(5):583-589.
22. Gilmour AG, Beckett HA. The voluntary reflux phenomenon. Br Dent J. 1993; 175(10):368-372.
23. Cortellini D, Parvizi A. Rehabilitation of severely eroded dentition utilizing all-ceramic restorations. Pract Proced Aesthet Dent. 2003;15(4):275-282.
24. Christensen GJ. The advantages of minimally invasive dentistry. J Am Dent Assoc. 2005;136(11):1563-1565.
25. White JM, Eakle WS. Rationale and treatment approach in minimally invasive dentistry. J Am Dent Assoc. 2000;131(9): 1250,1252.
26. Rainey JT. Understanding the applications of microdentistry. Compend Contin Educ Dent. 2001;22(11A):1018-1025.
27. Brantley CF, Bader JD, Shugars DA, Nesbit SP. Does the cycle of rerestoration lead to larger restorations? J Am Dent Assoc. 1995; 126(10):1407-1413.
28. Lutz F, Krejci I, Besek M. Operative dentistry: the missing clinical standards. Pract Periodontics Aesthet Dent. 1997;9(5):541-548.
About the Authors
Robert C. Margeas, DDS
Private Practice
Des Moines, Iowa
Adjunct Professor
Department of Operative Dentistry
University of Iowa College of Dentistry
Iowa City, Iowa
John Derango, DDS
Private Practice
LaSalle, Illinois
Clinical Instructor
The Kois Center
Seattle, Washinton