Zirconia and Computer-aided Design/Computer-aided Manufacturing (CAD/CAM) Dentistry
Gregg A. Helvey
Dental laboratory technology is evolving at a rapid pace. The use of zirconia as a metal substitute is a principal driving force behind the ever-increasing development and use of computerized dentistry. Most zirconia single copings and fixed partial denture (FPD) frameworks are milled from blocks of zirconia through the use of computer technology. This could explain the surge in the number of new computer companies entering the computer-aided design/computer-aided manufacturing (CAD/CAM) marketplace.
The burnout oven and casting machine have been replaced by model scanning and CAD/CAM milling machines. Keyboards, monitors, and cursors are used instead of Bunsen burners, wax, and carving instruments to fabricate crown-and-bridge frameworks.
Whether it is the recent dental school graduate or the seasoned dentist, today’s clinicians are bombarded with an overwhelming amount of information; this can be quite confusing, and not all manufacturer information may be 100% accurate. At times, it may seem less stressful to leave the decision about which dental material should be used to the laboratory technician, when, in fact, dentists are the ones rightly responsible for the prosthetic restorations placed in their patients’ mouths.
Consequently, dentists must be well-informed as to the type of dental materials they are prescribing for their patients, as well as their proper use and care. If zirconia is to replace metal, then it is the responsibility of the clinician to acquire as much knowledge as possible regarding the handling characteristics of the material. There are short-term zirconia-based successes reported,1-4 but further observation is still necessary. Unlike metal copings and multiple-unit frameworks that are virtually indestructible, zirconia has to be treated differently. The decision to use this material is based on improved esthetics and the patient’s desire to be metal-free, but it is imperative that the clinician understands the intricacies of zirconia.
Characteristics and Properties of Zirconia
Zirconia is the oxide of zirconium (Zr) metal found in nature combined with silicate oxide with the mineral name Zircon (ZrO2 SiO2) or Baddeleyite.5 Although it has been given the nickname "ceramic steel," the correct terminology is zirconia dioxide. It is technically incorrect to refer to the material as zirconia oxide, but the dental vernacular refers to it as zirconia, just as it uses alumina for the term alumina oxide and magnesia for magnesium oxide.6 Zirconia as a pure oxide does not occur in nature. The interest in using zirconia as a biomaterial is based on its mechanical strength, as well as its chemical and dimensional stability and elastic modulus similar to stainless steel.5 Zirconia has a normal density of 6 g/cm2, where the theoretical density (ie, 100% dense) of zirconium oxide is 6.51 g/cm2. The closer these two density values are, the less space between the particles, resulting in greater strength and a smoother surface7 (Figure 1 ).
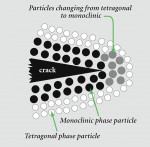
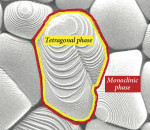
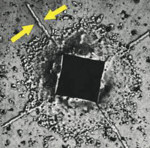
Depending on the temperature, zirconia can exist in three forms. Pure zirconia has a cubic structure at temperatures greater than 2,370° C. The cubic phase has a cubic form with square sides and moderate mechanical properties with a density of 6.27 g/cm2. The tetragonal phase exists at temperatures ranging from 1,170° C to 2,370° C. The tetragonal structure has a straight prism with rectangular sides and the most satisfactory mechanical properties with a density of 6.1 g/cm2. The monoclinic phase occurs at temperatures below 1,170° C8 and has a deformed parallelepipedonal (ie, a prism with six faces) shape, as well as the weakest mechanical properties with a density of 5.6 g/cm2 (Figure 2 ). In terms of strength, it is essential to limit the amount of the monoclinic phase because of its lower density. To stabilize zirconia at room temperature and control phase transformations, metal oxides, such as yttria (Y2O3) or ceria (CeO2), are added to the crystal structure.9 The addition of "stabilizing oxides" yields multiphase materials called partially stabilized zirconia.10 Technically, if yttria is added for stabilization, then it is referred to as yttria-stabilized tetragonal polycrystals (Y-TZP). A unique characteristic of zirconia is its ability to stop crack growth, which is "transformation toughening." An ensuing crack generates tensile stresses that induce a change from a tetragonal configuration to a monoclinic configuration and a localized volume increase of 3% to 5% (Figure 3 ). This volume increase results in a change of tensile stresses to compressive stresses generated around the tip of the crack (Figure 4 ). The compressive forces counter the external tensile forces and stop the further advancement of the crack11,12 (Figure 5). This characteristic accounts for the material’s low susceptibility to stress fatigue and high flexural strength of 900 MPa to 1,200 MPa.13,14
Fabrication of Zirconia Blanks
Zirconia is provided to dental laboratories in various sized blocks and can be milled into single-unit copings or multiple-unit fixed bridge frameworks. The zirconia blocks are fabricated in two basic ways.
Uniaxial Dry Pressing
This method involves applying pressure in a uniaxial direction to the ceramic powder confined in a mold.15 The irregular-shaped ceramic particles are pressed to a high "green" strength from interlocking and plastic deformation of the particles. This method’s major disadvantage is that the zirconia block will have varying degrees of density because of the particle/particle and mold wall/particle frictional effect. For example, depending on from which part of the block a three-unit fixed bridge framework is milled, the integrity of the framework could be compromised because of the different zones in the zirconia’s density. Usually these uniaxial dry-pressed blocks will be square or rectangular in shape.
Cold Isostatic Pressing
In this method, the zirconia powders are placed in a deformable mold that is subject to isostatic (ie, uniform in all directions) external pressure. This process results in a green-stage, chalk-like zirconia block with uniform density. Usually these blocks are cylindrical in shape. Regardless of the fabrication method, at this stage, the zirconia blocks are referred to as "green" blocks. These blocks can be further stabilized and densified (ie, 95% of theoretical density)11 through sintering in a special furnace without pressure. At this stage, the blank is referred to as a pre-sintered block. Further compression and heat can be applied to the blank, resulting in improved strength with complete densification.16 This process is referred to as hot isostatic postcompaction (HIP) and removes any residual porosity.16
The "green" zirconia blocks are milled at a larger dimension to compensate for 20% to 25% shrinkage during the sintering stage.12 Having the highest amount of porosity, these blocks are the fastest to mill and produce the least amount of wear on the milling machinery. In contrast, the HIP zirconia blocks (ie, "white" blocks) are milled at a 1:1 ratio because they are completely densified. Because of their increased hardness,11 these blocks are the slowest to mill and produce the most wear on the milling machinery. Various examples of green-stage block systems exist (Cercon, Degudent, Frankfurt, Germany; Lava, 3M™ ESPE™, St. Paul, MN; ZirkonZahn® USA, Atlanta, GA; and Hint-Els Zirkon TPZ-G, DigiDent, Girrbach, Pforzheim, Germany). There are also pre-sintered stage block systems (VITA In-Ceram® YZ Cubes, CEREC InLab, Charlotte, NC; KaVo EVEREST ZS-Blanks, KaVo Dental Corp, Lake Zurich, IL; Hint-Els Zirkon TZP-W, DigiDent; and DC-Shrink, POPP DCS, LLC, Greendale, WI) and HIP or completely sintered stage block systems (Denzir® Premium HIP Zirconia, etkon USA, Arlington, TX; Hint-Els Zirkon TZP-HIP, DigiDent; ZirKon™ Pro50™, Cynovad, Saint-Laurent (Quebec), Canada; and KaVo EVEREST ZH-Blanks, KaVo Dental Corp).
Zirconia Single-coping and Framework Fabrication Methods
There are three basic types of zirconia single-coping or fixed partial denture fabrication methods available today.
Manually Controlled System or Manual-aided Design/Manual-aided Manufacturing (MAD/MAM) Method
Referred to as copy milling, this method is based on the pantographic principle that was employed hundreds of years ago to copy or enlarge paintings, then later for engraving. The same principle is used at the hardware store when making duplicate keys. Using exact mechanical-tactile model surveying and analogous milling,17 it is considered to be highly precise in transfer accuracy. First, a coping or framework is manually fabricated in wax or composite, and then the pattern is placed into the pantographic machine. The copying arm of the machine traces the wax pattern while the cutting arm, which has a carbide cutter, mills a selected "green" or pre-sintered zirconia block. The final shape is 20% to 25% larger to account for shrinkage during the sintering step. The zirconia block has a density barcode label, so the copy mill machine can be adjusted properly to allow for shrinkage during the sintering phase.
Besides the lower cost factor for these types of milling machines (ZirkonZahn® USA; Ceramill, AmannGirrback Gmbh, Koblach, Austria; and TiZan™ Mill, Schutz Dental Group, Shelton, CT) this method of milling allows the dental technician to correct any discrepancies found in the tooth preparation by compensating during the waxing of the pattern.
Computer-aided Design/Computer-aided Manufacturing (Cad/Cam) Method
This second method of fabricating zirconia single copings and frameworks involves the manipulation of a 3-D design on the computer screen, followed by the automated production by a computer-controlled milling machine.18 There are three components to the CAD/CAM system: scanning, designing, and milling. All are usually housed at the laboratory.
Manufacturer-specific Closed System
The third method of fabrication used by dental laboratories is scanning the tooth-prepared models, designing the single coping or framework using a particular company’s construction software, and then electronically sending the information to the company’s milling center. This is usually a closed system, which means the scan and design data can only be used at that particular manufacturer’s milling center.
As stated earlier, milling "green" zirconia blocks takes the shortest amount of time and involves the least amount of wear and tear on the milling machines. In fact, milling machines designed to mill green-stage blocks are usually less expensive to acquire. On the other hand, milling "white" zirconia blocks (ie, fully sintered) takes more time, and the milling equipment is usually much more expensive to obtain. Furthermore, a number of these expensive milling machines are off-site from the dental laboratory.
Scanning Methods of Different Systems
The information obtained for the CAM phase will either come from the computer-aided design (CAD) phase, as seen with certain systems (Lava; KaVo EVEREST ZS-Blanks), or a scan of a wax/composite coping/framework (Cercon). Whatever system is used, a representation of the prepared teeth (ie, CAD/CAM) or wax-up of a coping or framework (ie, CAM only) must first be acquired and digitized onto the computer monitor.
Different systems use different scanning methods. These types of scanning methods include: optical cameras, contact digitization (ie, mechanical scanner), and white or colored light or laser projection.19,20 One chairside system (CEREC 3D, Sirona, Charlotte, NC) uses an intraoral optical camera to produce a digital image of the prepared tooth and the adjacent teeth. A charged-couple device (CCD) camera is employed in another system (Everest, KaVo Dental GmbH, Biberach, Germany) to produce a digital three-dimensional model generated by computing, recording, and merging dense-point clouds from 15 different angles and positions.19 Still another system (Procera®, Nobel Biocare, Yorba Linda, CA) reads the surface of a stone master die of the prepared tooth using a sphere or scanning stylus that is in contact with the stone surface. This information is electronically sent to one of the two milling centers for fabrication of the coping or framework. One particular system (Lava), for example, uses a laser optical system to digitize the master model. Not only are the tooth preparations digitized, but also all the edentulous areas between two bridge abutments.
CAD Software
Since the beginning, most dental CAD/CAM systems have been closed (eg, the CAD software information was specific to its CAM unit). The disadvantage to a closed system is that a laboratory, after making a significant monetary investment, is locked into whatever products the manufacturer’s CAM system provides. Today, however, more companies are opening their interfaces for industrial standard formats.21 For example, if a scan is made using the standard template library (STL) open file format, that data can be sent to any CAM system that accepts STL. This gives dental laboratories the freedom to choose different material suppliers and not be confined to one manufacturer’s technology. In one example of an open STL standard format (Dental Designer, 3Shape, Copenhagen, Denmark), the master die is scanned using a combination of laser lights and a high-resolution digital camera. Laser planes are pro-jected onto the master die and the camera captures the data. In general, CAD software allows the operator to design the appropriate dental prosthesis step by step. The software program mimics the same steps (ie, margin selection, block-out of any undercuts, and placement of die spacer for a cement layer) that the dental technician would follow to fabricate a single metal coping or a FPD framework. In the case of a FPD, the path of insertion is analyzed and, if necessary, the abutment is telescoped to optimize the insertion path. The data can then be electronically sent to a compatible off-site milling center.
CAM Hardware
The production of the zirconia coping or partial denture framework can be completed at the dental laboratory or at an off-site milling center. The actual fabrication can be accomplished using either a subtractive or an additive technique. The subtractive technique, which is the most commonly used, involves cutting the coping or framework from a solid block (Figure 6 ). The milling time and type of milling instruments used depend on the type of block (ie, green-stage, pre-sintered, or fully sintered) employed. The milled size of the coping or framework is also dependent on the amount of shrinkage that will occur during sintering. As noted earlier, sintering is required to achieve maximum strength for green-stage and pre-sintered blocks.
The additive technique involves building a coping or partial denture framework by adding material onto a die. When a zirconia product is desired, an oversized metal die must be created before the powder application to allow for shrinkage during the sintering process. As the powder is applied to the oversized metal die, it is compacted under isostatic pressure. At this "green" stage, a CAM milling procedure is used to finalize the outside contours of the coping or framework. The coping or framework is removed from the die and sintered at 1,550° C.
Selective laser sintering or melting is an alternative fabrication method that is presently producing metal frameworks and is in development for zirconia frameworks. Laser sintering involves the collection of the CAD data to create a 3-D freeform object. Fusing thin layers of a heat-fusing powder with a scanning laser beam creates a single coping or framework. Each scanned layer represents a mathematical cross section of the CAD model of a single coping or framework. The advantages of this type of fabrication are speed and lack of wasted material. This technology (BEGO Medifacturing® System, BEGO Medical GmbH, Bremen, Germany) is currently being tested in Europe, and plans are underway to introduce it in North America in 2008.
Tooth Preparation Parameters
The preparation of teeth is paramount to any restorative procedure. Without sufficient clearance, proper angulations, and visible finish lines, a dental technician will find it difficult to produce a quality restoration. Most CAD/CAM systems are highly sensitive to preparation discrepancies and offer little flexibility by the computer technician. With most systems, clear finish lines, lack of parallel axial walls, rounded internal line angles, and lack of undercuts are necessary to produce CAD/CAM restorations.22 On the other hand, CAM-only and copy milling systems, as previously mentioned, allow for some flexibility to compensate for any discrepancies that may compromise the fabrication of the coping/framework. For example, by telescoping an abutment during the waxing phase, a compromised path of insertion can be remedied for a FPD framework.
Either way, there are certain recommendations for preparing teeth when using zirconia as a substrate. There are similar recommendations shared by all systems, as well as specific recommendations that are dependent on the particular CAD/CAM system used for coping/framework fabrication.
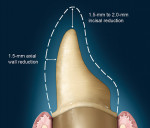
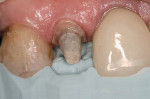
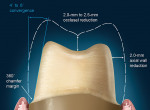
The shared preparation principles in the anterior and posterior regions for all systems (ie, single-unit and FPD abutment) include an incisal/occlusal reduction of 1.5 mm to 2 mm23 (Figure 7). These are the minimal amounts of reduction that are acceptable. Increasing the amount of incisal reduction in the anterior region to 2 mm gives the dental technician more room to develop the esthetics. A silicone index of the mock-up study model can be used to verify the proper reduction (Figure 8). In the posterior region, increasing the occlusal reduction to 2.5 mm provides space to create natural, unworn anatomy24 (Figure 9). A 2-mm occlusal clearance tab (Flexible Clear-ance Tabs™, Kerr Sybron, Orange, CA; Occlusal Reduction Ring, Clinician’s Choice Dental Products, Inc, New Milford, CT) should be used to confirm that sufficient reduction has been achieved.
Most CAD/CAM and copy milling systems recommend that the minimum axial wall reduction for anterior crowns be 1 mm to 1.5 mm, and 1 mm to 2 mm for posterior crowns. Certain manufacturers, however, will have other requirements. For instance, if a particular manufacturer-specific system (Procera) is used as the substructure, the manufacturer states that a minimum axial reduction of 0.8 mm is all that is required. The total occlusal convergence angle can range between 4° to 6°.25 An optical scanner requires a minimum of a 4° occlusal convergence angle to adequately read the margin of the master die.23,26 Parallel walls "confuse" most scanners and should be avoided.
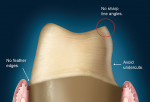
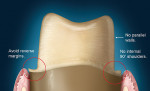
The internal aspect of the margin should be either a rounded shoulder or a deep chamfer of at least 1 mm.23 A 90° internal angle is contraindicated. The exit angle of the gingival margin should be a butt joint and lack any beveled edges. Knife-edge or feather margins are not acceptable because they do not allow for adequate areas for porcelain build-up. Undercuts and sharp line angles should be avoided, also (Figure 10). Sharp line angles should be rounded to avoid overmilling by the diamond-cutting bur. When sharp line angles remain on the occlusal axial surface, the milling bur, which has a specific diameter, will remove excessive material to copy the sharp line angle. The overmilling can result in structurally compromised areas (Figure 11).
Trough or gutter margins and parallel axial walls should be avoided because they may prevent accurate scanning of the preparation (Figure 12). The margin can be finished with a flat-ended, safe-sided diamond bur, which will eliminate any unevenness or crests that can develop during preparation. In the case of FPD abutments, the walls of the prepared teeth need to be tapered relative to the path of insertion. If the individual walls are tapered but parallel in relation to each other, this results in a compromised scan path. This scenario may not enable the axial wall to be properly scanned and will result in a loose-fitting framework or open margins.
Clinical Fitting of Zirconia Core Crowns
Permanent placement of any restoration always requires a try-in step to confirm the fit and marginal integrity. Clinically, situations occur when some adjustment is necessary to achieve complete seating of the restoration. The adjustment can be made on the prepared tooth or to the internal surface of the restoration. In a case where the clinician adjusts the prepared tooth, an indicating mechanism can be employed to determine the location of the "high spot" that is preventing full seating of the restoration.
For example, a super-thin (ie, 1/1,500 of an inch) single-sided articulation paper (Accufilm® I, Parkell Inc, Edgewood, NY) can be placed (ink side to the tooth) between the restoration and the prepared tooth. When the restoration is placed with finger pressure, an indication of the "high spot" is revealed. After providing anesthesia, the clinician can correct the discrepancy and confirm the fit. If necessary, the same steps should be repeated.
The alternative method of restoration fit adjustment is to grind, usually with a diamond or carbide cutting instrument, the internal aspect of the restoration. This is a common method with metal substrate restorations and usually presents no problems. In the case of a zirconia core restoration, fit adjustment using a diamond-cutting instrument can alter the physical properties of the zirconia-based core and the clinician should be aware of these effects.
Grinding Effects on Zirconia CoresIn the literature, the effect of surface grinding on zirconia is contradictory and is related to the volume percentage of transformed zirconia which, in turn, depends on the metastability of the t -> m phase transformation, the grinding severity, and the locally developed temperatures.27-31 Grinding can increase the strength of Y-TZP zirconia.32 Grinding produces microcracks that induce surface transformation toughening. This transformation results in the development of compressive strains from the transformation-related volume increase several microns below the surface.33 Subsequently, microcrack propagation is prevented by the surface compressive strains resulting in an increase of the flexural strength of the zirconia.32
On the other hand, deep surface flaws develop that act as stress concentrators and decrease the flexural strength if their depth exceeds the grinding induced surface compressive layer.27 Kosmac et al found that surface grinding and sandblasting demonstrated a counteracting effect on the strength of Y-TZP ceramics. Dental grinding lowered the mean strength and Weibull modulus, whereas sandblasting provided a powerful method for strengthening, but at the expense of somewhat lower reliability.
Sandblasting was found to be more effective than grinding in inducing the t -> m transformation and, therefore, increasing the mean flexural strength of the ceramic.28
Guazzato et al examined the influence of sandblasting, grinding, grinding orientation, polishing, and heat treatment on the flexural strength of Y-TZP.8 Their study suggested that sandblasting and grinding may be recommended to increase the strength of dental YTZP, provided they are not followed by heat treatment. Fine polishing may remove the layer of compressive stresses and, therefore, lower the mean flexural strength.8
Curtis et al investigated the influence of surface modification techniques before final cementation of a restoration, namely alumina abrasion and surface grinding routinely used by clinicians.34 They found that the combination of the reduced surface roughness and the formation of a surface layer of compressive stress as a result of the alumina abrasion increased the reliability of the biaxial flexure strength. On the other hand, course grinding significantly reduced the biaxial strength and the Weibull modulus because of the increased surface roughness.34
Further studies are necessary to investigate the long-term effects of surface modification of zirconia cores before a definitive treatment protocol is established for all clinicians. For now, several manufacturers strongly recommend not exceeding 30 psi when sandblasting. A number of dental technicians who are presently using different zirconia systems suggest that the preferred method for fit adjustment is altering the tooth preparation rather than the zirconia substrate. The main reason is inadvertent damage (ie, microcracks, heat, coping perforation) to the zirconia substrate that can lead to catastrophic failure. The exception would be if the "high spot" was present on the tooth margin. The adjustment then would be performed on the zirconia substrate.
Clinicians should exercise caution when adjusting zirconia cores. Kosmac et al27,28 found that grinding with a coarse-grit diamond bur mounted on a high-speed handpiece used at 150,000 rpm caused local development of temperatures that exceeded the temperature where the reverse m -> t transformation occurs. As previously stated, tetragonal phase transformation to the monoclinic transformation increases the flexural strength. Localized heat development can reverse the transformation and decrease the flexural strength. Swain and Hannink35 demonstrated that grinding with a 91-µm grit size diamond wheel (at 3,300 rpm) under water coolant favored the retention of the monoclinic phase, maintaining the flexural strength. It should be understood that keeping this monoclinic phase is not desirable because if further stress is applied, failure could result. Similar to a small crack in a windshield, the crack will grow over time with continued vibration.
The size of the diamond grit is important in abrasive grinding. As the diamond grit size increases, so does the rate of material removal.36 However, removal rate/grit size is not necessarily linear. Yin et al found that the removal rate for porcelain was higher by about 15% with coarse diamond burs; however, the removal rate with coarse diamond burs for zirconia increased nearly 50%.37 The use of coarse diamond burs can increase the propensity for subsurface cracks with concomitant strength degradation.38 When adjusting zirconia cores, most dental technicians use either a high-speed handpiece with a water coolant or a slow-speed handpiece with light pressure and a uniform grit diamond bur. The shape and tightness of the diamond cutting bur affects the surface roughness and depth of the flaws, which influence the flexural strength.39 One particular line of diamond cutting instruments is specifically made for cutting zirconia (Magic Touch, Strauss Diamond Instruments, Inc, Westport, CT). These diamond burs were designed to be used in the CAM milling machines. The patented unique diamond grit was developed to cut hard ceramics and help prevent microfractures in the zirconia (Figure 13).
Cementation of Zirconia-based Restorations
Methods for cementing zirconia-based restorations can be divided into two groups: conventional and adhesive cementation. Often used in the literature, lectures, and commercial product handouts, the broad term "conventional cementation" can imply that any nonadhesive cementation technique may be used to insert zirconia-based restorations. In fact, some articles include the use of glass ionomer-based cements and zinc phosphate.26,40 This method includes conditioning the tooth, if required, then seating the restoration under pressure. In its technical bulletin, 3M ESPE states not to use light-curing glass ionomer cements because of water absorption, which may cause the cement to expand after placement. This is because of the fact that different resin-modified, glass-ionomer cements demonstrate different absorption coefficients, and some of them exceed the tolerance for zirconia. The various absorption coefficients are supported in a study conducted by Kanchanavasita et al.41 The United Kingdom-based Renishaw Company, a leading provider of metrology and spectroscopy that also has a dental division that fabricates zirconia copings/frameworks, does not recommend the use of resin-modified, glass-ionomer cements with zirconia-based cores.
In their study of two different resin-modified, glass-ionomer cements, Huang et al42 found extensive hygroscopic expansion. Creating artificial gaps, they placed resin-modified, glass-ionomer cement in glass cylinders. Cracking of 40% of the glass cylinders was found after 2 weeks, and of 70% after 4 weeks.42 Sindel et al43 evaluated the effects of various luting materials on the crack formation of all-ceramic crowns. They concluded that the hygroscopic expansion of resin-modified, glass-ionomer cement led to failure of all-ceramic crowns. On the other hand, Snyder et al44 conducted studies to determine if hygroscopic expansion of resin-modified, glass-ionomer cement would cause fracture of sintered alumina copings of all-ceramic crowns. The results showed no fracture of the all-ceramic coping as a result of expansion of the cement alone.44 Most recently, Raigrodski et al1 monitored 20 three-unit posterior zirconia-based FPDs over a mean follow-up of 31.2 months and found that they all performed well during this short-term service. At this point, further investigation is necessary to establish the long-term effects of using specific resin-modified, glass ionomers to cement zirconia-based cores.
Adhesive bonding is the other method of cementing zirconia-based restorations. This method can be further subcategorized into self-etching luting cements (RelyX™ Unicem, 3M ESPE; Embrace™ Wetbond™, Pulpdent® Corporation, Watertown, MA) and traditional bonding/luting cements. The former are used simply by mixing the cement, loading it, and placing the restoration. A study by Goracci et al45 did find that interfacial strength and adaptation of self-etching and self-adhesive dual-curing cements were enhanced if a seating force greater than finger pressure was maintained throughout the initial self-curing period.
According to manufacturers’ instructions, there is no tooth conditioning required before cementation with self-etching adhesive cements. However, Hitika et al46 found a negative influence on the bond strength of a self-etching luting cement (RelyX™ Unicem) to enamel where no phosphoric etching was performed before cementation.
The other subcategory is the traditional adhesive bonding method that uses a three-step, a two-step, or a one-step bonding protocol. There are numerous studies showing the effectiveness of adhesive bonding to enamel and dentin. One crucial step in effective resin bonding is surface conditioning of the ceramic surface with hydrofluoric acid.
Traditional adhesive bonding has the ability to restore 100% of the flexural strength of the tooth with a porcelain laminate veneer, provided the ceramic is etched with hydrofluoric acid and is bonded to enamel within a 3:1 cement ratio.47-50 In the case of zirconia, hydrofluoric acid produces no effect on the bonding surface because there is no glass to etch.
Palacios et al51 found no significant difference between three different luting agents (Panavia F 2.0 and ED Primer A&B, Kuraray America, Inc, New York, NY; RelyX™; and RelyX Unicem) upon removal stress application. The crowns were removed using a mean removal stress ranging from 5 MPa to 6.1 MPa. In 46% of the specimens, the mode of failure was cement remaining on the zirconium oxide coping. Cement was found on the tooth in 25.7% of the specimens. Therefore, surface preparation may increase the bond strength of resin to the zirconia surface before using a traditional adhesive protocol. In lieu of hydrofluoric acid etching, tribochemical silica coating (CoJet™ Sand and Rocatec, 3M ESPE) produces an adhesive bonding surface that permits the use of traditional bonding materials.52,53
Atsu et al found that tribochemical silica coating and the application of a 10-methacryloyloxydecyl dihydrogen phosphate monomer (MDP)-containing bonding/silane coupling agent mixture has been shown to increase the shear bond strength between zirconium-oxide ceramic and resin luting agent (Panavia F 2.0 and ED Primer.54
Surface Preparation Effects on Cementing/Bonding Zirconia-based Restorations
There have been several studies investigating the bond strength and durability of various bonding methods to high-strength oxide ceramics.55 There is, however, some controversy as to the proper surface conditioning before cementation. Air abrasion, which is commonly used in combination with phosphate monomer (MDP)-containing luting cements, has been shown to produce a strong and durable bond.56,57 Zhang et al showed a decrease in fracture resistance of airborne alumina-abraded (ie, sandblasted) specimens compared to polished specimens.58 Rekow and colleagues59 achieved excellent bond strengths without sandblasting; they instead treated the bonding surfaces with metal primers (Panavia 21, Kuraray America) in combination with adhesive cement (RelyX Unicem). These metal primers were Alloy Primer (Ap-MDP/VBATDT-containing monomer) or Epiguard (E-MDP-containing monomer).60 However, Wolfart et al found that roughening and activating the zirconia surface by air abrasion before adhesive bonding and the use of a MDP-containing resin composite (Panavia F 2.0 and ED Primer A&B) was necessary to achieve durable bonding to densely sintered zirconia ceramics.55 As previously mentioned, tribochemical silica coating is another consideration for surface treatment. The surface preparation of the bonding surface of, for example, a porcelain veneer is standardized. The commonly accepted procedure is to etch the bonding surface with hydrofluoric acid, silanate the surface, and then use a resin luting agent for cementation. Porcelain veneers have more than 20 years of history.
Zirconia-based restorations, at this point, do not have a lengthy history and the various surface treatments and luting materials for their placement need further investigation.
Conclusion
The use of computer technology in dentistry is becoming a more integral part of how clinicians restore the human dentition. CAD/CAM technology, although relatively new to dentistry, has been used in the automotive and aerospace industry for much longer. The development of accurate scanning devices, expansion of software capabilities, precision milling in the use of CAD/CAM technologies, and newer nonmetal materials make dentistry one of the fastest-changing disciplines. These technological advances reduce laboratory hours and inherent flaws produced though man-made methods, while providing a more esthetic product than metal. Restricted only by the human element, computers present an unlimited range of function and possibilities for dental clinicians now and in the future.
References
1. Raigrodski AJ, Chiche GJ, Potiket N, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006;96: 237-244.
2. Sailer I, Fehér A, Filser F, et al. Prospective clinical study of zirconia posterior fixed partial dentures: 3-year follow-up. Quintessence Int. 2006;37:685-693.
3. Sturzenegger B, Fehér A, Lüthy H, et al. [Clinical study of zirconium oxide bridges in the posterior segments fabricated with the DCM system]. Schweiz Monatsschr Zahnmed. 2000;110:131-139.
4. Vult von Steyern P, Carlson P, Nilner K. All-ceramic fixed partial dentures designed according to the DC- Zirkon technique. A 2-year clin-ical study. J Oral Rehabil. 2005;32:180-187.
5. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1-25.
6. Cramer von Clausburch S. Zirkon and Zirkonium. Dent Lab. 2003;51: 1137-1142.
7. Duran P, Moure C. Sintering at near theoretical density and properties of PZT ceramics chemically prepared. J Mater Sci. 20:827-833.
8. Guazzato M, Quach L, Albakry M, et al. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J Dent. 2005;33:9-18.
9. McLaren EA, Giordano RA. Zirconia-based ceramics: Material properties, esthetic and layering techniques of new veneering porcelain, VM9. Quintessence Dent Tech. 2005;28:99-111.
10. Helvey GA. Press-to-zirconia: a case study utilizing cad/cam technology and the wax injection method. Pract Proced Aesthet Dent. 2006;18:547-553.
11. Christel P, Meunier A, Heller M, et al. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23:45-61.
12. Raidgrodski AJ. Contemporary all-ceramic fixed partial dentures: a review. Dent Clin North Am. 2004;48:viii, 531-544.
13. Hauptmann H, Suttor D, Frank S, et al. Ma-terial properties of all-ceramic zirconia prosthesis. J Dent Res. 2000;19:507.
14. Roundtree P, Nothdurft F, Pospiech P. In-vitro investigations on the fracture strength of all-ceramic bridges of ZrO2- ceramic [abstract]. J Dent Res. 2001;80:57.
15. Rogers J, Weber W. Ceramic materials are not all the same. Spectrum Dialogue. 2007;6:76-80.
16. Li J, Liao H, Hermansson L. Sintering of partially-stabilized zirconia and partially-stabilized zirconia-hydroxyapatite composites by hot isostatic pressing and pressureless sintering. Biomaterials. 1996;17:1787-1790.
17. Reichert A, Herkommer D, Müller W. Copy milling of zirconia. Spectrum Dialogue. 2007;6:40-56.
18. Tinschert J, Natt G, Hassenpflug S, et al. Status of current CAD/CAM technology in dental medicine. Int J Comput Dent. 2004;7:25-45.
19. Liu PR. A panorama of dental CAD/CAM restorative systems. Compend Contin Educ Dent. 2005;26:507-508, 510, 512.
20. Witkowski S. CAD-CAM in dental technology. Quintessence. 2005:1-16.
21. Riquier R. Rapid manufacturing what will be next. Sprectrum Dialogue. 2007;6:116-120.
22. Kurbad A. Clinical aspects of all-ceramic CAD/CAM restorations. Int J Comput Dent. 2002;5:183-197.
23. Raigrodski AJ. Clinical and laboratory considerations for the use of CAD/CAM Y-TZP-based restorations. Pract Proced Aesthet Dent. 2003;15:469-476.
24. McLaren EA, Hyo L. CAD/CAM update: Technologies and materials and clinical perspectives. Inside Dentistry. 2006;Nov/Dec:98-103.
25. Doyle MG, Munoz CA, Goodacre CJ, et al. The effect of tooth preparation design on the break-ing strength of Dicor crowns: 2. Int J Prosthodont. 1990;3:241-248.
26. Boudrias P. The yttrium tetragonal zirconia polycrystals(Y-TZP) infrastructure: The new chapter in the search for metal framework replacement. J Dent Quebec. 2005;42:172-176.
27. Kosmac T, Oblak C, Jevnikar P, et al. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent Mater. 1999;15:426-433.
28. Kosmac T, Oblak C, Jevnikar P, et al. Strength and reliability of surface treatedY-TZP dental ceramics. J Biomed Mater Res. 2000;53: 304-313.
29. Gupta PK. Strengthening by surface damage in metastable tetragonal zirconia. Journal of the American Ceramic Society. 1980;63: 117-21.
30. Green DJ. A technique for introducing surface compression into zirconia ceramics. Journal of the American Ceramic Society. 1983;66: C178-C179.
31. Swain MV. Limitation of maximum strength of zirconia-toughened ceramics by transformation toughening increment. Journal of the American Ceramic Society. 1985;68:C97-C99.
32. Luthardt RG, Holzhüter MS, Rudolph H, et al. CAD/CAM-machining effects on Y-TZP zirconia. Dent Mater. 2004;20:655-662.
33. Garvie RC, Hannink RH, Pascoe RT. Ceramic steel? Nature. 1975;258:703-704.
34. Curtis AR, Wright AJ, Fleming GJ. The influence of surface modification techniques on the performance of a Y-TZP dental ceramic. J Dent. 2006;34: 195-206.
35. Swain MV, Hannink RHJ. Metastability of the martensitic transformation in a12mol%ceria-zirconia alloy: grinding studies. Journal of the American Ceramic Society. 1989;72: 1358-1364.
36. Siegel SC, Von Fraunhofer JA. Dental cutting: the historical development ofdiamond burs. J Am Dent Assoc. 1998;129: 740-745.
37. Yin L, Jahanmir S, Ives LK. Abrasive machining of porcelain and zirconia with a dental handpiece. Wear. 2003;255:975-989.
38. Jahanmir S, Xu HHK, Ives LK. In: Jahanmir S, Koshy P, Ramulu M, eds. Mechanism of material removal in abrasive machining of ceramics. Machining of Ceramics and Composites. NY: Marcel Dekker; 1999:11-84.
39. Luthardt RG, Holzhüter M, Sandkuhl O, et al. Reliability and properties of ground Y-TZP-zirconia ceramics. J Dent Res. 2002;81:487-491.
40. de Lima Navarro MF, Santos MJ, Mondelli RF, et al. Cementation considerations for CAD/CAM all-ceramic restorations. Pract Proced Aesthet Dent. 2004;16: 550-551.
41. Kanchanavasita W, Anstice HM, Pearson GJ. Water sorption characteristics of resin-modified glass-ionomer cements. Biomaterials. 1997;18:343-349.
42. Huang C, Kei LH, Wei SH, et al. The influence of hygroscopic expansion of resin based restorative materials on artificial gap reduction. J Adhes Dent. 2002;4:61-71.
43. Sindel J, Frankenberger R, Krämer N, et al. Crack formation of all-ceramic crowns dependent on different core build-up and luting materials. J Dent. 1999;27:175-181.
44. Snyder MD, Lang BR, Razzoog ME. The efficacy of luting all-ceramic crowns with resin-modified glass ionomer cement. J Am Dent Assoc. 2003;134: 609-612.
45. Goracci C, Cury AH, Cantoro A, et al. Microtensile bond strength and interfacial properties of self-etching and self-adhesive resin cements used to lute composite onlays under different seating forces. J Adhes Dent. 2006;8:327-335.
46. Hikita K, Van Meerbeek B, De Munck J, et al. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dent Mater. 2007;23:71-80.
47. Magne P, Kwon KR, Belser UC, et al. Crack propensity of porcelain laminate veneers: A simulated operatory evaluation. J Prosthet Dent. 1999;81: 327-334.
48. Magne P, Versluis A, Douglas WH. Effect of luting composite shrinkage and thermal loads on the stress distribution in porcelain laminate veneers. J Prosthet Dent. 1999;81:335-344.
49. Magne P, Douglas WH. Porcelain veneers: dentin bonding optimization and biomimetic recovery of the crown. Int J Prosthodont. 1999;12: 111-121.
50. Addison O, Marquis PM, Fleming GJ. Resin elasticity and the strengthening of all-ceramic restorations. J Dent Res. 2007;86:519-523.
51. Palacios RP, Johnson GH, Phillips KM, et al. Retention of zirconium oxide ceramic crowns with three types of cement. J Prosthet Dent. 2006;96:104-114.
52. Matinlinna JP, Heikkinen T, Ozcan M, et al. Evaluation of resin adhesion to zirconia ceramic using some organosilanes. Dent Mater. 2006;22:824-831.
53. Xible AA, de Jesus Tavarez RR, de Araujo Cdos R, et al. Effect of silica coating and silanization on flexural and composite-resin bond strengths of zirconia posts: An in vitro study. J Prosthet Dent. 2006;95: 224-229.
54. Atsu SS, Kilicarslan MA, Kucukesmen HC, et al. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J Prosthet Dent. 2006;95:430-436.
55. Wolfart M, Lehmann F, Wolfart S, et al. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater. 2007;23:45-50.
56. Blatz MB, Sadan A, Martin J, et al. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004;91:356-362.
57. Wegner SM, Kern M. Long-term resin bond strength to zirconia ceramic. J Adhes Dent. 2000;2:139-147.
58. Zhang Y, Lawn BR, Rekow ED, et al. Effect of sandblasting on the long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004;71:381-386.
59. Rekow ED. Dental CAD/CAM systems: a 20-year success story. J Am Dent Assoc. 2006;137 Suppl:5S-6S.
60. Dias De Souza GM, Silva N, Goes M, et al. Effect of metal primers on cementbonds to fully sintered zirconia. "https://iadr.confex.com/iadr/2006Orld/techprogram/abstract_75149.htm". 2006; Abstract #324.
This article was adapted from Functional Esthetics and Restorative Dentistry. 2007;1(3):28-39.
About the Author
Theodore P. Croll, DDS
Private Practice
Pediatric Dentistry
Doylestown, Pennsylvania
Affiliate Professor
Department of Pediatric Dentistry
University of Washington
School of Dentistry
Seattle,Washington
Adjunct Professor
Pediatric Dentistry
University of Texas Health Science
Center at San Antonio
San Antonio, Texas
John W. Nicholson, PhD
Professor of Biomaterials Chemistry
Department of Chemical
Environmental and Pharmaceutical Sciences
School of Science
University of Greenwich,Medway Campus
Chatham, Kent, United Kingdom