CAD/CAM Fabricated Crowns Used to Esthetically Mask Teeth Stained by Tetracycline
Richard Trushkowsky
Appearance has become an important aspect of dentistry in recent years as evidenced by the rapid growth of bleaching products and the success of total makeover shows.1,2 Patients may desire to change their smile because of a variety of factors including shape, imperfections (such as chips), size, wear, and color of the existing dentition. A comprehensive diagnosis requires radiographs, photographs, and study models. The selection of the appropriate treatment will depend on the patient’s desires and the dentist’s determination of the best technique to satisfy the patient’s needs. Treatment of tooth discoloration will be emphasized in this article.
The correct diagnosis of the cause of discoloration is paramount as it may effect the final treatment. Teeth possess a number of colors, and each tooth will usually be darker at the gingival aspect than the incisal edge because of the thinness of the enamel in this area.1 Canines are usually darker than the central or laterals. Aging is also a cause of darkness due to the laying down of secondary dentin, extrinsic stain, and wear of the enamel. The color of the tooth is best assessed with a standardized light source1 such as the Rite-Lite LED Shade Matching Light (AdDent, Inc, Danbury, CT), which provides 5500° K light for shade matching, or the Demetron Shade Light (Kerr Corporation, Orange, CA) to reduce the effects of metamerism.
The coronal portion of the tooth consists of enamel, dentin, and the pulp. Any changes manifested in these components will change a tooth’s appearance caused by light’s transmitting and reflecting properties. Discoloration can be intrinsic, extrinsic, or internalized.2 Temporary stains, such as food dyes, tobacco, and alcohol, are easily removed by a prophylaxis. Extrinsic discolorations existing only in the enamel layer may have been caused by hypoplasia, fluorosis, and aging. White or brown spots are usually caused by hypoplastic enamel. Excessive fluoride intake during tooth formation can result in mottled enamel. Enamel permeability increases with age, allowing staining to occur more readily. Older enamel is thinner and more translucent, permitting the darker dentin to be more visible. A variety of metabolic diseases, systemic factors, and local factors (such as injury) can affect the developing dentition and result in discoloration. Amelogenesis imperfecta, dentinogenesis imperfecta, fluorosis, tetracycline staining, and pulpal hemorrhagic products are examples of this phenomenon. Internalized discoloration can be a result of either developmental or acquired defects such as tooth wear and restorative materials. Intrinsic discoloration can be caused by trauma, endodontic therapy, metallic restorations, and tetracycline.1
In the early 1960s, it was determined that tetracycline use could result in tooth discoloration.3 Tetracycline becomes bound to calcified tooth structure if it is ingested during the calcification stage of tooth development. The mineralization of the permanent dentition is not complete until 8 years of age. Tetracycline forms complexes with calcium ions on the surface of hydroxyl apatite crystals within bone and dental tissue; this occurs more in dentin than enamel. Tetracycline crosses the placental barrier and should be avoided from 29 weeks in utero until full term. The color change depends on the medication used and the dosage over time. Tetracycline and oxytetracycline both cause a yellow discoloration. Chlortetracycline results in a grey-brown discoloration. Minocline is a semi-synthetic tetracycline derivative that is often used to treat acne vulgaris and rosacea. Staining may result in the adult dentition of 3% to 6% of patients on a long-term course of minocycline therapy. The discoloration may start from the first month to many years after treatment is initiated. The precise mechanism is still under investigation.2
Various techniques have been used to address the discoloration caused by tetracycline ingestion, including long-term nightguard bleaching, chairside bleaching,4 porcelain veneers,5 and crowns. The use of ceramic restorations is the most predictable for its lasting effect. Opaque porcelains or opaque luting cements are sometimes incorporated.6 Unfortunately, their use often creates a very bright white and a dull artificial manifestation that looks unnatural. Color neutralization using a complementary color to the stain is the best way to achieve a more natural appearance. This can be accomplished through the selection of the luting material or various porcelain modifiers. The value of the restoration has to be increased to achieve the final effect.7,8 Another technique of masking the color is the use of an opaque core in the ceramic. Aluminum oxide, lithium disilicate, or zirconium can be used.9,10
The zirconia11-16 material used by most manufacturers is a tetragonal polycrystalline zirconia, partially stabilized by yttrium oxide. This structure is referred to as a transformation-toughened material and has the special property of inhibiting fractures. Tensile stresses acting at the crack tip induce a transformation of the metastable tetragonal zirconium-oxide phase into the thermodynamically more favorable monoclinic form. This transformation is associated with a local increase in volume. This will result in com-pressive stresses generated at the tip of the crack which counteract the external stress acting on the crack tip.12,14 This leads to high initial strength and fracture toughness and low susceptibility to stress fatigue. The strength values allow lower frame thicknesses of 0.3 mm to 0.5 mm. This permits less aggressive tooth reduction compared to other systems.
There are varieties despite the same chemical composition. There are differences in the strength and translucency depending on the powder type and production condition. Some zirconia materials have a strength of 900 MPa while others have strengths over 1,100 MPa. Some zirconia materials have bright white opaque color while others do not. Cercon® Zirconia (Dentsply Ceramco, York, PA) and DCS-Precident (DCS Dental AG Allschwill, Switzerland) use a white-colored core; however, Lava™ (3M ESPE, St. Paul, MN) uses a yttria-tetragonal zirconia polycrystal (Y-TZP) core which is more translucent but can be colored to match the VITA® Lumin shade guide (Vident, Brea, CA). This is useful as the veneering porcelain can be eliminated in areas where interocclusal space is limited (lingual of maxillary anteriors).
The Y-TZP frameworks can be designed by wax-ups or CAD/CAM. Cercon uses the conventional waxing techniques and DCS-Precident and Lava incorporate different CAD technology. Cercon and Lava used partially sintered blanks that are processed by the CAD/CAM system. IPS e.max® ZirCAD blocks (Ivoclar Vivadent®, Amherst, NY) are made of yttrium-oxide–stabilized zirconium oxide. In the partially sintered, chalk-like state, IPS e.max ZirCAD can be easily milled in the inLab unit (Sirona Dental Systems, Charlotte, NC). The milling process is conducted by enlarging the framework by 20%. A specially designed fluorescent zircon liner provides an optimum bond and is used to apply the basic shade on the frameworks. Subsequently, IPS e.max ZirPress is pressed in a fully or partially anatomical manner onto the crown copings and bridge frameworks, or IPS e.max Ceram layering ceramic is used to apply the veneers. DC-Zirkon copings (DCS Dental AG) are milled from fully sintered Y-TZP–based blanks.
Zirconia can be formed by two processes. The green machining approach is achieved by using a spray-dried zirconia powder. This powder is isostatically pressed, pre-sintered, and a holder is used to mount it into the CAD/CAM system. It is milled in this pre-sintered state and then sintered pressureless in a furnace at 1,350° C to 1,500° C. Milling fully sintered zirconium oxide blanks may induce cracks and damage the microstructure of the zirconium oxide. The porous pre-sintered zirconia is initially enlarged but shrinks by approximately 20% linear after sintering. Hot isostatic pressing (HIP) is another technique using a closed system, high temperature, and pressure to densify the material. This allows a 20% gain in strength over a non-HIP zirconia. However, this material is difficult to mill and needs to be sprayed with water and slowly milled so that cracks are not induced. Ceramic restorations fail as a result of surface tension. Cracks and flaws propagate by slow growth of the crack until the applied load exceeds the load-bearing capacity of the restoration. Ultimately, catastrophic failure occurs.
The Lava system uses a framework with a veneering ceramic that is fabricated specifically to be used together. They should not be combined with other materials. The Lava system’s range of veneer ceramics is coordinated to the shades which are applied to the Lava system framework.15-18 No light-absorbent opaquer or opaque dentin layer is necessary for the build-up of Lava all-ceramic restorations.
Case Presentation
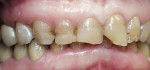
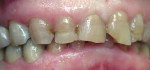
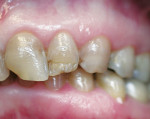
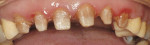
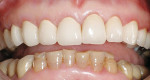
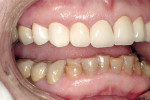
A 45-year-old woman presented with a chief complaint of dark, chipped, short, and malformed anterior teeth (Figure 1, Figure 2, Figure 3). The patient had fixed porcelain-fused-to-metal bridges in the upper right and lower right quadrants with single crowns in the lower left quadrant. Photographs, full-mouth radiographs, and study models were obtained. Examination of the mounted models, photographs, and clinical records demonstrated a deep overbite, collapsed vertical dimension of occlusion, loss of canine guidance, and wear of the anterior teeth with super-eruption of the lower anterior teeth. The gingival heights of the maxillary teeth were uneven. The suggested treatment plan included restoration of her vertical dimension of occlusion with restorations, periodontal surgery to correct gingival heights, and osseous contour. Treatment could also have included a possible intrusion of the lower anterior teeth and restoring them with porcelain veneers after more incisal space was created. However, the patient’s finances and busy work schedule did not allow her to proceed with more comprehensive treatment. She elected to have teeth Nos. 4 through 13 restored with Lava crowns to mask the dark teeth. She was informed that neglecting her other problems may compromise the longevity of the restorations and lead to further breakdown.
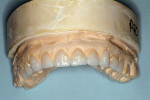
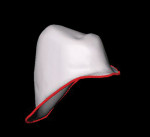
The laboratory provided a diagnostic wax-up of the projected restorations (Figure 4). The teeth could have been made longer if the vertical had been restored. The laboratory provided a preparation guide to check for adequate facial/buccal (Figure 5 and Figure 6) and incisal/occlusal reduction (Figure 7). An index of the diagnostic wax-up could be used to fabricate provisionals after the teeth were prepared (Figure 8). Teeth Nos. 5 through 13 were prepared for Lava crowns (Figure 9), and full-mouth impressions were obtained. Provisional restorations provided by the laboratory were then relined and temporarily placed in the patient’s mouth. The patient was given the opportunity to assess the appearance, function, and phonetics of the final restorations. At the next visit, the teeth were isolated and the crowns were cemented with PANAVIA (Kuraray America, Inc, New York City, NY) after receiving the patient’s approval (Figure 10, Figure 11, Figure 12, Figure 13). Impressions were obtained for a nightguard and a lower bleaching tray. The nightguard was subsequently inserted but the patient did not proceed with lower bleaching tray.
Preparation
The optimal preparation is a shoulder or chamfer with a circumferential step or chamfer which must be applied at a horizontal angle of > 5°. The vertical angle of the preparation should be 4° or larger. The inside angle of the shoulder preparation must be given a rounded contour. A feather-edge preparation is contraindicated as it does not provide adequate reduction for the porcelain build-up or the trough shoulder because the outer edge may not be detected when scanned.
Laboratory Fabrication
The laboratory should pour the model with a colored type IV or V die stone (white, beige, buff, or green) without plastic additives. A light, dull (non-reflective) surface works best for the scanning procedure. The model bases should be flat and have minimal thickness and size to ensure optical accessibility. All segments of the saw-cut model must be removable from the base with a double, triple, or block-pin die system to prevent rotation or movement of the die(s) and tissue (pontic) areas. Dies should be ditched directly below the margin. The margins should not be marked and no die spacer or hardener should be placed on the die preparation area. This would cause a reflective surface on the die that will compromise the scanned data of the die surface. Undercuts should be blocked out or based out with a resin block-out material or light-colored wax.14
The sectioned model is then positioned in the Lava Scan, which is a PC-based system. The preparations are automatically scanned and then displayed on the monitor as a three-dimensional (3-D) image (Figure 14A and Figure 14B). The tooth preparations, antaglio surfaces, and the opposing casts are scanned and used to create a virtual design of the framework. The space needed for the luting material is incorporated into the design. Block out of any remaining undercuts can also be incorporated.
After the design is completed, the suitable zirconia block with its unique plastic holder and barcode is selected. This design of the unit is registered with the barcode. If a bridge was being fabricated, the pontic and connections can be inserted using the keyboard and mouse and displayed on the monitor (Figure 15). The mandatory minimal size of the connectors is 9 mm. A true CAD/CAM system, a wax-up is not required with the Lava system. The data is then transferred to the Lava form milling unit and then framework coloring is accomplished according to the prescription. The average milling time for a coping is 35 minutes, and 75 minutes for a three-unit bridge. The individual units or framework is then sintered for approximately 8 hours. Twenty percent shrinkage occurs during sintering and the CAD process allows for a 20% expansion. Then the selected shade of veneering porcelain is applied and fired. The frameworks are available in eight shades and the veneering porcelain is available in 16 VITA® Classic shades. A variety of intensive materials, incisal materials, enamel effect materials, and transparent materials allow the technician to achieve the outcome described in the dentist’s prescription.14
Luting
The internal surface of Procera® AllZirkon (Nobel Biocare USA Inc, Yorba Linda, CA) has a rough surface caused by the manufacturing process. This roughness may create a micromechanical attachment of resin cement. Traditional silica-based ceramics can be acid-etched and treated with a silane coupling agent to increase wettability. However, high-strength aluminum oxide and zirconium oxide ceramics are not silica-based and have to be treated differently. Kern and Wegner19 found that air abrasion and the use of PANAVIA21 containing 10-methacryloyloxydecyl dihydrogen phosphate (MDP) provided a durable bond to zirconium oxide. Blatz and coworkers20 found that the ceramic material and internal surface for the several commercial zirconia systems are different.21-23 The same adhesive technique may result in different results with each system. The study seemed to indicate that the application of a MDP-containing bonding/silane coupling agent is the cause of the consistent bond to Procera AllZirkon. Air abrasion with Al2O3 increases surface energy and wettability. However, the microporosities that are created may act as crack initiators and weaken the restoration. On the other hand, resin luting materials may “heal” minor surface flaws and significantly strengthen the ceramic material. Long-term water storage and thermal cycling are the best way to simulate intraoral conditions that stress the bonded surfaces. Zhang and colleagues22 found that damage by particle abrasion can reduce the strength of Y-TZP ceramics, and fatigue testing demonstrated a reduction in strength of 20% to 30%. Trace moisture greatly increased the damage. Various crack modes can occur, resulting in crown failure depending on the thickness of the ceramic and porcelain layers.
RelyX™ Unicem (3M ESPE) has demonstrated higher bond strengths than PANAVIA after water storage and thermocycling. The compressive strength and microhardness of RelyX Unicem were also found to be higher than PANAVIA. Kumbuloglu et al23 found that air abrasion caused a transformation of the monoclinic phase of zirconia, resulting in a layer of compressive stresses on the surface that counteracts flaws caused by air abrasion. Light-curing glass ionomer cements should not be used because water absorption may cause the cement to expand after placement, resulting in fracture of the restoration.
The variety of zirconia materials available respond differently to various luting protocols. Conventional cementation with glass ionomer or resin cement without air abrasion is usually all that is necessary. Subgingival preparations that cannot be isolated should be cemented with glass ionomer cement.24-28
Conclusion
There has been a strong desire for a non-metallic restorative material that could be used both in the anterior and posterior for single crowns and for three- to four-unit bridges. Ceramics provide biocompatibility, color stability, high wear resistance, low thermal conductivity, and excellent esthetics. However, ceramics are brittle because of atomic bonds that do not allow atomic planes to slide apart upon being loaded.27 This is an important point in selecting the appropriate ceramic material. Zirconia Y-TZP cores are glass-free, as they have a polycrystalline microstructure. With glass systems there is a decomposition of the glass structure and increased crack propagation as saliva reacts with the glass in the system (stress corrosion). This does not happen with zirconia Y-TZP. However, tensile failure between the veneer of relatively weak porcelain may still be a problem.28-31 Finishing and grinding of the sintered zirconia are both contraindicated especially in the connector area. The concentration of heavy stresses in the connector areas increase the risk of failure; therefore, an abutment should not have increased mobility.18
Heavy bruxers who have severe parafunctional activity that cannot be controlled are not good candidates for all-ceramic bridgework. If proper guidelines in case selection, preparation, and cementation are followed, Lava and other zirconia restorations should provide years of service and excellent esthetics. It is especially useful in masking any underlying discoloration as in the case presented.
Acknowledgment
The author would like to thank Adrian Jurim, MDT, of Jurim Dental Studio for the excellent laboratory work, and 3M ESPE for the graphic slides.
References
1. Watts A, Addy M. Tooth discoloration and staining: a review of the literature. Br Dent J. 2001;190(6):309-316.
2. Tredwin CJ, Scully C, Bagan-Sebastian JV. Drug-induced disorders of teeth. J Dent Res. 2005;84(7):596-602.
3. Lambrou DB, Tahos BS, Lambrou KD. In vitro studies of the phenomenon of tetracycline incorporation into enamel. J Dent Res. 1977;56(12):1527-1532.
4. McCaslin AJ, Haywood VB, Potter BJ, et al. Assessing dentin color changes from nightguard vital bleaching. J Am Dent Assoc. 1999;130(10):1485-1490.
5. Feinman RA, Madray G, Yarbough D. Chemical, optical, and physiologic mechanisms of bleaching products: a review. Pract Periodont Aesthet Dent. 1991;3(2):32-36.
6. Okuda WH. Using a modified subopaquing technique to treat highly discolored dentition. J Am Dent Assoc. 2000;131:945-950.
7. Meyenberg K. Current challenges in esthetic dentistry: Restoring discolored teeth and missing adjacent teeth. Quintessence Dent Technology. 2002;25: 110-116.
8. Ahmad I. Masking tooth discoloration with all-ceramic restorations. Quintessence Dent Technology. 2004;27:9-18.
9. McLean JW. Evolution of dental ceramics in the twentieth century. J Prosthet Dent. 2001;85(1):61-66.
10. Raptis NV, Michalakis KX, Hirayama H. Optical behavior of current ceramic systems. Int J Periodontics Restorative Dent. 2006;26: 31-41.11.
11. Luthardt RG, Holzh¦ter M, Sandkuhl O, et al. Reliabilty and properties of ground Y-TZP-Zirconia ceramics. J Dent Res. 2002;81(7):487-491.
12. Sorensen JA. The Lava system for CAD/CAM production of high strength precision fixed prosthodontics. Quintessence Dent Technology. 2003;(26):57-67.
13. Sorensen JA. The Lava™ all-ceramic system: CAD/CAM Zirconia prosthodontics for the 21st century. Synergy in Dentistry. 2003;2(1):3-6.
14. 3M ESPE Lava™ All-Ceramic System Technical Product Profile.
15. Hedge TK. Achieving clinical and esthetic success by placing a Zirconia-based all ceramic three-unit anterior fixed partial denture. Synergy in Dentistry. 2003;2(1): 7-11.
16. Trinkner TF, Roberts M. Placement of an all-ceramic, three unit posterior bridge fabricated with esthetic and durable zirconium oxide connectors. Synergy in Dentistry. 2003;2(2): 3-7.
17. Raigrodski AJ. Clinical and laboratory considerations for achieving function with aesthetic with the Lava system. Spectrum International. 2003:1-5.
18. Raigrodski AJ. Contemporary all-ceramic fixed partial dentures: a review. Dent Clin North Am. 2004;48(2):531-544.
19. Kern M, Wegner SM. Bonding to zirconia ceramic: adhesion methods and their durability. Dent Mater. 1998;14(1):64-71.
20. Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89(3):268-274.
21. Blatz MB, Sadan A, Kern M. Adhesive cementation of high-strength ceramic restorations: Clinical and laboratory guidelines. Quintessence of Dental Technology. 2003;26:47-55.
22. Zhang Y, Lawn BR, Malament K, et al. Damaging accumulation and fatigue life of particle-abraded ceramics. Int J Prosthodont. 2006;19: 442-448.
23. Kumbuloglu O, Lassila LV, User A, Vallittu PK. Bonding of resin composite to zirconium oxide by two air-particle abrasion methods. Oper Dent. 2006;31(2):248-255.
24. Matinlinna JP, Heijjinen T, Ozcan M, et al. Evaluation of resin adhesion to zirconia ceramic using some organosilanes Dent Mater. 2006;22(9):824-831.
25. Ernst CP, Cohnen U, Stender E, Willerhausen B. In vitro retenitive strength of zirconium oxide ceramic crowns using different lutig agents. J Prosthet Dent. 2005;93(6): 551-558.
26. Palacios RD, Johnson GH, Phillips KM, Raigrodski AJ. Retention of zirconium oxide ceramic crowns with three types of cement. J Prosthet Dent. 2006;96(2): 104-114.
27. Ottino MA, Valandro LF, Scotti R, et al. Effect of surface treatments on the resin bond to zirconium-based ceramic. Int J Prosthodont. 2005;18(1):60-65.
28. Atsu SS, Kilicarslan AMA. Kucukesman HC, et al. Effect of zirconium oxide ceramic surface treatment on the bond strength to adhesive resin. J Prosthet Dent. 2006;95(6):430-436.
29. Aboushelib MN, de Jager N, Kleverlaan CJ, et al. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dent Mater. 2005;21(10):984-991.
30. Vult von Steyern PV, Ebbesson S, Holmgren J, et al. Fracture strength of two oxide ceramic crown systems after cyclic pre-loading and thermocycling. J Oral Rehabil. 2006;33(9):682-689.
31. White SN, Miklus VG, McLaren EA, et al. Flexural strength of a layered zirconia and porcelain dental all-ceramic system. J Prosthet Dent. 2005;94(2): 125-131.
32. Poitiket N, Chiche G, Finger IM. In vitro fracture strength of teeth restored with different all-ceramic crown systems. J Prosthet Dent. 2004;92(5):491-495.
33. Itinoche KM, Ãzcan M, Bottino MA, Oyafuso D. Effect of mechanical cycling on the flexural strength of densely sintered ceramics. Dent Mater. 2006;22(11):1029-1034.
About the Author
Richard Trushkowsky, DDS
Private Practice
Staten Island, New York