PRGF— Plasma Rich in Growth Factors: A Simple Solution for Post-extraction Sites
Immediate gratification finds new meaning for the “Baby Boomer” population—those 50+ year-olds who lead busy lives and careers and demand service, luxury, and immediate load implants. This is the same group who wishes to retain their youth and in whom the hands of the healing clock are slowed. Today, the challenge of satisfying their needs and wants becomes simplified with the use of plasma rich in growth factors (PRGF), a gel-like substance prepared from two to four 5-cc vials of the patient’s own blood that have been subjected to a 8-minute centrifugation cycle at 460g, then meticulously pipetted into plasma fractions that contain the growth factors targeted for regeneration of both hard and soft tissue (Figure 1).1
Of the growth factors (GFs) most abundant in PRGF are 3 whose actions most significantly affect this regenerative process. PDGF increases the proliferation of osteoblasts in vitro; TGF- in specific doses boosts type I and type IV collagen-derived protein synthesis as well as increases mineralization of the bone matrix, favoring implant anchorage. Insulin-like growth factor-I (IGF-I) stimulates bone formation, inducing cellular proliferation, differentiation, and biosynthesis of type I collagen in addition to its mitogenic effect on multinucleated osteoclastic cells.1 More importantly, the unique combination of GFs produced with this protocol works synergistically. PRGF differentiates itself from other platelet-rich plasmas (PRPs) because it intentionally excludes inflammatory leukocytes; moreover, PRGF’s activation with calcium chloride alone forbids the use of thrombin because it shocks platelets. (The use of bovine thrombin is prohibited in Europe.2) Instead, the PRGF activation protocol yields a gradual-release kinetics for precious healing proteins stored in the alpha-granules.
Current treatment protocols3 recommend a routine 6- to 12-month course of healing; this requirement increases the risk of reabsorption of the alveolar process and loss of crestal height and width that, in some cases, can delay, jeopardize, or even preclude implant placement. Others work reasonably well on soft tissue regeneration but fail with bone regeneration.4,5 Intervention with PRGF in the same surgery as the extraction can derail the physiological inevitability of further crestal bone loss and improve the quality and quantity of bone regenerated. Such a scenario is possible thanks to the PRGF technique, which dramatically hastens the healing process in post-extraction sites, as well as grafting for periapical lesions, 3-wall defects, large craters around teeth, gingival recession and, in some cases, can enable immediate implant placement which would have been delayed otherwise.6-10 Another favorable clinical observation is that over 10 years in 5,000 post-extraction sites filled with PRGF in this practice, there has not been even one incident of alveolitis (dry socket).
The following case typifies increased quantity and bone density quality as well as healthy soft tissue. Results illustrated are at 12 to 16 weeks.
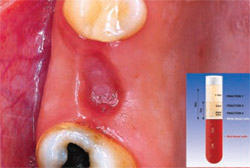
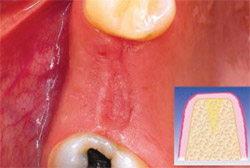
PRGF was prepared by drawing 4 vials of blood from the peripheral vein of the forearm into sterile 0.5-mL tubes containing sodium citrate as an anticoagulant. The tubes were tilted gently to disperse the anticoagulant, then placed symmetrically into buckets in a specially programmed centrifuge (BTI Biotechnology Institute, Vitoria, Spain) which separates whole blood into gradients. Figure 2, a schematic of an extraction site, represents the area to be filled. Fraction 3— the richest in GFs—was used to graft the defect itself (Figure 3), while Fraction 1—the poorest in growth factors—was used to prepare the fibrin membrane to cover the extraction site. In this case, no additional grafting material was required. Figure 4 shows the extraction at 24 hours.
The uppermost fraction (F1) was pipetted and transferred into a glass receptacle and placed on top of a heating block (Heating Block, BTI Biotechnology Institute) for approximately 25 minutes at 37°C (98.6°F) to produce autologous fibrin to seal the extraction site (Figure 5). Note the progression of healing at 3 months (Figure 6). Accelerated epithelialization can be attributed to the regenerative properties of the specific growth factors contained in the clot produced from Fraction 3 (Figure 7). At 4 months, the large defect, including the vestibular table, has been regenerated (Figure 8).
The patient achieved a very satisfactory functional and aesthetic solution (Figure 9), and demonstrated excellent implant stability at 1 year after placement (Figure 10).
References
1. Anitua, E, Andía I. PRGF (Plasma Rich in Growth Factors). Dental Dialogue. 2004:III.2. Weibrich G, Kleis WK, Hitzler WE, et al. Comparison of the platelet concentrate collection system with the plasma-rich-in growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005;20(1):118-123.
3. Marx RE, Carson ER, Eichstaedt RN, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral MedOral Pathol Oral Radiol Endod. 1998;85(6):638-646.
4. Lemons PP, Chen D, Whitehart SW. Molecular mechanisms of platelet exocytosis:Requirements for alpha-granule release. Biochem Biophys Res Comm. 2000; 267(3):875-880.
5. Whitman, DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55(11):1294-1299.
6. Anitua E. Platelet-derived growth factors: their utilization in the preparation of future sites for implants. Paper presented at: Annual Reunion of the Spanish Society of Periodontists; November, 1997; Alicante, Spain.
7. Anitua, E. Platelet rich plasma. Growth factor enhancement for developing future sites. Paper presented at: XIII Annual Meeting Academy of Osseointegration; March, 1998; Atlanta, GA.
8. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-535.
9. Anitua E. A new approach in bone regeneration. Plasma rich in growth factors (PRGF). September 2000 Puesta al Día Publicaciones, S.L.
10. Anitua E, Ardanza B, Paponneau A, et al. Clots from platelet-rich plasma promote bone regeneration and in so doing reduce the time needed for dental implants and favoring their osseointegration. Blood. 2001;11:242a.
This article was written by Eduardo Anitua, DDS, MD
Disclaimer: The safety and effectiveness of this device for use in in vivo indications has not been established by the FDA.
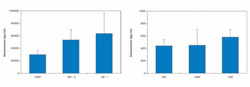 | ||
| Figure 1 Concentrations of the principal GFs contained in PRGF. Note the concentrations of IGF-I, TGFβ and PDGF. | ||
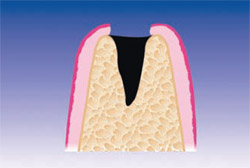 | 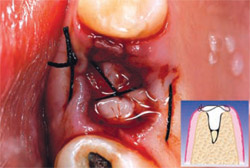 | |
| Figure 2 Post-extraction alveolus. | Figure 3 Alveolus filled with PRGF clot and a fibrin membrane produced with the same protocol. | |
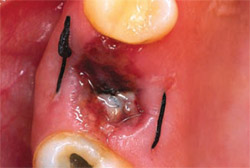 |  | |
| Figure 4 The extraction site at 24 hours. | Figure 5 The fibrin membrane used to cover the PRGF-filled extraction site is produced from F1, which takes about 30 minutes to form after its activation. At 15 days, it has facilitated epithelialization. Healing time can vary between 5 and 15 days depending on the size of the alveolus and on the health of the patient. | |
 |  | |
| Figure 6 At 3 months, the soft tissue demonstrates excellent healing. | Figure 7 A PRGF clot is produced from the plasma fraction richest in growth factors (F3) immediately above the white blood cells; it forms between 6 to 7 minutes after activation. | |
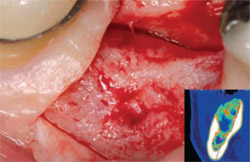 | 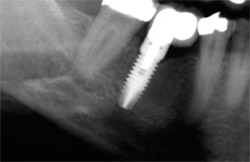 | |
| Figure 8 At 16 weeks, note that the large defect (12 mm x 16 mm) has healed nicely. BTI Scan® (inset) reveals how the total loss of the vestibular table is being regenerated. | Figure 9 Rx 1 year after implant placement. | |
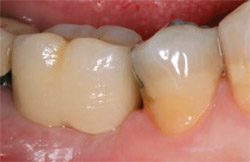 | ||
| Figure 10 The final restoration. |



