Case Presentations Demonstrating Periodontal Treatment Variation: PEARL Network
Abstract:
BACKGROUND: Variation in periodontal terminology can affect the diagnosis and treatment plan as assessed by practicing general dentists in the Practitioners Engaged in Applied Research and Learning (PEARL) Network. General dentists participating in the PEARL Network are highly screened, credentialed, and qualified and may not be representative of the general population of dentists.
METHODS: Ten randomized case presentations ranging from periodontal health to gingivitis, to mild, moderate, and severe periodontitis were randomly presented to respondents. Descriptive comparisons were made between these diagnosis groups in terms of the treatment recommendations following diagnosis.
RESULTS: PEARL practitioners assessing periodontal clinical scenarios were found to either over- or under-diagnose the case presentations, which affected treatment planning, while the remaining responses concurred with respect to the diagnosis. The predominant diagnosis was compared with that assigned by two practicing periodontists. There was variation in treatment based on the diagnosis for gingivitis and the lesser forms of periodontitis.
CONCLUSION: Data suggests that a lack of clarity of periodontal terminology affects both diagnosis and treatment planning, and terminology may be improved by having diagnosis codes, which could be used to assess treatment outcomes.
CLINICAL IMPLICATIONS: This article provides data to support best practice for the use of diagnosis coding and integration of dentistry with medicine using ICD-10 terminology.
With the advent of electronic medical records, consistency of terminology would create a better referral relationship between general dentists, periodontists, and other healthcare providers, improving transparency and communication and, ultimately, benefitting patients in treatment. When dentistry was exempt from reporting diagnosis codes with billing data (HIPAA, 1996), this enabled the diagnostic system to grow with a lack of clarity around diagnosis.1 Several systems of diagnosis exist in dentistry (eg, SNOWDENT, EZ codes, ICD-9-CM, ICD-10), however, no one system is the clear choice until mandated.2-7
It has been previously reported in the literature that dentistry lacks consensus in defining and curating periodontal terms related to oral diagnosis, and how this affects treatment planning.8 The established guidelines for diagnosis in periodontitis as stated by Armitage and the parameters of care documents from the American Academy of Periodontology (AAP) exhibit more than 12 distinct discrepancies between the aforementioned systems and the mandated medical diagnostic coding system in the United States.2,8-11 Variation in terminology has been found to impact diagnosis and may contribute to the scientific translational gap, in this case between specialists and general practitioners.4,9 Both the patient and the profession could benefit from a consensus in terminology to address the variation in diagnostic criteria for chronic periodontitis. Table 1 provides a summary of current periodontal terminology definitions from the AAP as well as others that have been published in the literature.2,8-12
Having its origins based in surgery, dentistry has continually limited itself by being defined by treatment procedures instead of the patient’s diagnosis. This limitation may become a distinct disadvantage with the use of electronic health records. The disconnect between diagnosis and procedure codes, exacerbated by the variation in periodontal terminology, has been previously reported to affect periodontal diagnosis.13 This article extends the findings of a previously reported study to demonstrate that treatment planning recommendations can be affected by variation in terminology defining diagnosis.8
Dentistry is currently based on a disease management system rather than a health model of dental care delivery.8 International Classification of Disease (ICD) diagnosis codes are part of the medical coding process, and the latest revision, ICD-10-CM (10th revision, Clinical Modification), is the basis for the electronic medical home concept, providing an opportune time for dentistry to collaborate with medicine for improved patient outcomes. Additionally, as defined in the Affordable Care Act,14 many pediatric hospital-based dental clinics will integrate dental records with electronic medical records using ICD-10 coding.7 Congress has delayed the start date for the ICD-10-CM until the fall of 2015; until then, Congress mandates that medical providers in the United States code with the ICD-9-CM (9th revision, Clinical Modification).7
The Practitioners Engaged in Applied Research and Learning (PEARL) Network is a practice-based research network (PBRN) initially supported by the National Institute of Dental and Craniofacial Research/National Institutes of Health (NIDCR/NIH) and built upon the principles of Good Clinical Practice (GCP). PEARL Network dentists are termed practitioner-investigators (P-Is), and the Network currently conducts translational studies and is no longer dependent on federal funding. Currently, the PEARL Network has a registry of approximately 300 P-Is throughout the continental United States.8,15 PEARL P-Is are screened, credentialed, and qualified prior to becoming a member and may not be representative of the general population of dentists at large.15,16 This article describes the PEARL P-Is detailed clinical assessment of case scenarios and treatment approaches to various periodontal conditions and provides insight into how procedure codes can limit patient treatment. The purpose of this study was to provide a foundation for calibration of the PEARL Network for future clinical periodontal studies.
Periodontitis is one of the most common chronic oral health conditions affecting worldwide populations and remains a health burden in the US population.17,18 The prevalence of periodontitis has been reported with different systems of classification and even different data collection standards over the past 50 years.13,19 The challenge with periodontal diagnosis is that both health and disease can exist simultaneously. Dentists chart specific disease sites and report a variation of periodontal health. For instance, a patient may have a localized disease condition with inflammation, pocketing, and radiographic bone loss, which complicates the diagnosis when based on procedures, causing variation in reporting. Specific diagnostic classification criteria to address missing values (Table 1) and clearly define diagnosis in chronic periodontitis may improve oral healthcare, and might be accomplished through a consensus meeting to define terminology.
Materials and Methods
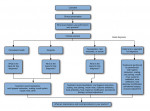
A sample of PEARL Network dentists completed an online survey of case scenarios using a proprietary 41 Code of Federal Regulations (CFR)–approved electronic data capture system, AdvantageEDCSM (The EMMES Corporation, www.emmes.com), supported by the PEARL data coordinating center at The EMMES Corporation, Rockville, MD. Survey respondents provided informed consent prior to participation, and Institutional Review Board (IRB) approval was obtained by the New York University School of Medicine prior to study initiation. There were a total of 10 different clinical scenarios with conditions ranging from periodontal health to severe periodontitis. Each survey respondent was randomly presented five of the 10 clinical scenarios in what the authors termed randomized case presentations (RCPs). Figure 1 depicts the flow of the study, order of RCP, and survey questions.
Clinical presentations were described, and respondents provided information on the examinations they would perform and the information they would chart if presented clinically. The clinical scenario was then described as an overall patient summary with characteristics of conditions presented, and each respondent made a diagnosis and was then asked the basis of that diagnosis. The respondents could make a diagnosis of periodontal health, gingivitis, or periodontitis (mild, moderate, or severe). Alternatively, they could refer to a specialist for the diagnosis. Depending on the diagnosis made, each respondent was asked to select the appropriate treatments. Product names were collected for all over-the-counter and prescription drugs recommended for treatment. All participants were then asked about maintenance and recall practices once periodontal treatment planning selections were completed for that particular clinical scenario.
Practitioners’ diagnoses for each clinical scenario were categorized as: 1) under-diagnosis: those who selected less severe diagnosis; 2) concurrence: those who selected what was considered the correct response (as defined by “predominant” or “periodontist assigned” diagnosis); and 3) over-diagnosis: those who selected a more severe case type than what was considered correct. The “predominant” diagnosis response for a particular scenario was the one that the majority of the respondents selected. The results were also compared to a “periodontist assigned” diagnosis determined by input from a number of clinical and research periodontists and finalized by two practicing PEARL periodontists (JM and RC), one of which is board-certified (RC). The cases were developed with a team of periodontal research experts and approved by the NIDCR protocol review committee.
Results
Of the 132 survey respondents, two were excluded because they were specialists, thus the results reported here are based on 130 general dentists. Table 2 describes the diagnosis by PEARL dentists for the randomized case scenarios. Table 3 through Table 6 present treatment recommendations by diagnosis. Note that all clinical scenarios where the respondent would refer to a specialist for diagnosis are excluded, as is the one scenario where the respondent did not answer this survey question. In the case of missing data for a particular treatment option, percentages presented are based on all available data.
Table 3 summarizes the treatment recommendations for the clinical scenario in which the correct diagnosis was gingival health for both the “predominant” diagnosis and the “periodontist assigned” diagnosis. Dentists who concurred for this clinical scenario (56.9%) selected the diagnosis of periodontal health. Those who over-diagnosed (43.1%) selected the diagnosis of gingivitis or mild periodontitis for this clinical scenario.
As shown in Table 3, when the respondent made a diagnosis of health, the treatments prescribed were oral hygiene instruction (94.6%), scaling (78.4%), full-mouth polish (97.3%), and mouth rinse (24.3%). When an over-diagnosis was made, the treatments prescribed were oral hygiene instruction (100%), scaling (92.6%) and mouth rinse (50%).
Table 4 summarizes the treatment recommendations of the clinical scenario in which the correct diagnosis was gingivitis for both the “predominant” diagnosis and the “periodontist assigned” diagnosis. Dentists who under-diagnosed this clinical scenario (9.4%) selected health as the diagnosis, while those who concurred (54.7%) made a diagnosis of gingivitis. Those who over-diagnosed this clinical scenario (35.9%) selected periodontitis (mild, moderate, or severe). Regardless of the diagnosis made by the survey respondent, treatment recommendations almost always included oral hygiene instruction, scaling, and full-mouth polish. Mouth rinse was recommended less often when the clinical case presentation was under-diagnosed and more often when there was an over-diagnosis.
Table 5 summarizes the treatment recommendations for the eight clinical scenarios in which the correct diagnosis was periodontitis. Two of the eight cases diagnosed with periodontitis did not agree between the “predominant” diagnosis and the “periodontist assigned” diagnosis. Of the 471 completed clinical scenarios with a correct diagnosis of periodontitis using the “predominant” diagnosis, 110 (23.4%) under-diagnosed, 331 (70.3%) concurred, and 30 (6.4%) over-diagnosed. Note that the severity of periodontitis is included in the definition of under-diagnosis, concurrence, and over-diagnosis. The 45 instances where the respondent would refer to a specialist for diagnosis are excluded. Responses were categorized as: under-diagnosed (23.4%) when a diagnosis of health, gingivitis, or a less severe form or periodontitis was made with the “predominant” diagnosis; concurred with the “predominant” diagnosis (70.3%); and over-diagnosed (6.4%) when a more severe form of periodontitis was selected. The proportion of recommendations did not differ across levels of diagnostic agreement for the following: oral hygiene instruction, scaling, and mouth rinses. Several treatments were more common when the respondent correctly diagnosed the patient or over-diagnosed: root planing, local antibiotics, periodontal surgery, and occlusal adjustment. Interestingly, two treatments were recommended more often for a correct diagnosis than for both under- and over-diagnoses: systemic antibiotics and host response modification.
Lastly, Table 6 summarizes the treatment recommendations by the respondent’s actual diagnosis regardless of what the correct diagnosis was for that particular scenario. Oral hygiene instruction is almost always recommended (96.2% to 100%) and would most commonly be delegated to a hygienist. Scaling was performed for the majority of clinical scenarios (79.2% to 98.6%), particularly when gingivitis or periodontitis diagnoses were made, and would most commonly be performed by a hygienist. Adjunctive therapy with a mouth rinse represented the most variation by diagnosis, ranging from 24.5% for healthy gingiva to 82.3% for severe periodontitis. Full-mouth polish was only a treatment option when a diagnosis of health or gingivitis was made; it would be recommended the majority of the time for both diagnoses and usually performed by the hygienist. Root planing was recommended (93.9% to 97.2%) and would usually be done by a hygienist, however for severe periodontitis it was also just as likely to be performed by a periodontist. Systemic antibiotics were recommended less often but increased to 32.6% when severe periodontitis was diagnosed. Local antibiotics were recommended slightly more often and also increased with severity of the diagnosis, ranging from 26.4% for mild periodontitis to 42.1% for severe periodontitis. Host response modification was rarely recommended for mild periodontitis (9.2%) but more often for severe periodontitis (31.9%). Periodontal surgery would be recommended for a mild periodontitis diagnosis 6.7% of the time and for moderate periodontitis 36.6% of the time, but most respondents would recommend periodontal surgery for severe periodontitis (74.5%). For the majority of times periodontal surgery was recommended, it was indicated that it should be performed by a periodontist. Occlusal adjustment was recommended more often than periodontal surgery for mild periodontitis and increased as the severity of the diagnosis increased. Occlusal adjustment was usually performed by the dentist.
Chlorhexidine formulations were the most common mouth rinses prescribed by dentists in the PEARL Network for diagnoses of periodontitis, followed by mouth rinse with essential oils. Minocycline hypochloride microspheres were the most recommended local antibiotic for treatment of periodontitis; a low-dose doxycycline was the most recommended host response modifier for treatment of periodontitis.
Discussion
The authors have taken the variation in periodontal terminology to its clinical endpoint of treatment planning and have demonstrated that this variation is not in the best interests of the patient and fails to optimize the general practitioner/periodontist referral mechanism.8 The study describes a detailed approach by PEARL dentists on how thorough they are in examining a patient and in recording clinical information. Clinical studies can be evaluated by investigator attention to detail and patient compliance especially for follow-up visits in a randomized controlled study. PEARL Network P-Is achieved a very high percentage of compliance in follow-up visits for randomized controlled trials (RCTs), which is consistent with the data presented on quality and provider examination subroutines. Specifically, attendance at the three follow-up visits for an RCT of non-carious cervical lesions was 98.9%, and the follow-up rate for the two post-treatment visits in a study of postoperative hypersensitivity in Class I and II carious lesions was 98.7%.20 Initial screening of practitioners to ensure data integrity cannot be underestimated. The profile of PEARL practitioners is representative of a highly motivated dentist interested in conducting office-based clinical studies, which the authors estimate represents, at best, some 3% to 5% of dentists. However, their responses may reflect the best-case scenario, whereas in the general population of dentists non-concurrence would most likely be higher.
Implications of over-diagnosis and under-diagnosis may increase cost, both in the short and long term. Over-diagnosis may result in more costly procedures and interventions. Under-diagnosis may result in lack of necessary procedures and interventions, thus exacerbating the condition. For two scenarios there was a difference between the “periodontist assigned” diagnosis determined by two practicing periodontists and the “predominant” diagnosis by dentists of the PEARL Network. The “predominant” diagnosis was more severe in both instances. Procedure codes can limit practitioners’ ability to optimally treat patients and may cause them to fit the diagnosis into a compartment or procedure influenced by reimbursement. Ideally, procedure codes state the procedure and avoid imbedding the diagnosis into the definition. The philosophy of defining diagnosis within certain procedure codes can, at times, challenge the ethics of a practice by dictating care. Table 3, Table 4, and Table 5 reference codes where the diagnosis is imbedded in the code; by using the term “preventive” or “periodontal disease” as part of the American Dental Association’s (ADA) Current Dental Terminology (CDT) code descriptor, the diagnosis of health or periodontitis is implied. While CDT procedure codes were not in this study, they are presented to demonstrate the discrepancy between practice and coding that may influence diagnosis and treatment of cases.
This study presents a detailed assessment of how PEARL general dentists diagnose and treat periodontal conditions. Dentists practice to respond to treating diseases with a set of given procedures as established by the ADA’s CDT.21 The evolving model of healthcare is to view the patient in the context of health rather than disease. A model that is based on procedures may limit the ability of practitioners to manage their patients’ overall health and well-being. This complicates the new patient-centered approach of assessing clinical outcomes. Current treatment plans respond to the site-specific conditions and do not allow the dentist to include health-related approaches such as risk assessment, prevention, and the overall management of the clinical outcomes. Diagnosis codes would allow dentists more latitude to assess “the patient” rather than limiting their assessment to site-specific treatments and thus provide a broader base of treatment. This point of view affords the opportunity for dentistry to integrate into the patients’ total healthcare system, a philosophy that has been discussed in the profession for decades. PEARL Network dentists represent practitioners who bill and code treatments in accordance with current dental terminology treatment codes curated and maintained by the ADA.
Over-diagnosis of periodontal disease adds to the cost of healthcare. Under-diagnosis of periodontal disease progression could hinder early intervention efforts and subsequent less invasive and costly therapy. This can be illustrated with the under-utilization of a mouth rinse that carries a label claim for the use in gingivitis.19 The data suggests an increased market potential for the use of labeled pharmacotherapy to treat periodontitis.
Root planing and subgingival scaling should be defined terms for their prescribed use as therapeutic or prophylactic, or in some cases both, depending upon the diagnosis.8,22 For treatment to coincide with the individual patient needs, the current system needs calibration and standardization of diagnosis. The level of detail to incorporate risk assessment as an outcome needs to be recognized. Third-party coverage currently focuses payment on treatment rather than assessment. To optimize patients’ oral health, the authors suggest care should be managed in the overall context of total patient health and well-being consistent with patient-centered care. The present findings support diagnosis coding and increased reimbursement for clinical assessments by dentists, which may be a distinguishing characteristic that will separate dentists from mid-level practitioners.
Currently, adjunctive therapies for treatment of gingivitis and periodontitis include local delivery of antibiotics.20 The cost of treatment in the new healthcare model will be based on clinical outcomes, rather than procedures. Dentistry is not currently mandated to record diagnosis when treatment planning.1 However, diagnosis is a part of every dentist’s decision to treat and is the basis for designing the patient’s treatment plan.20 The P-Is’ use of a mouth rinse is an example of the proper utilization of label claims; as severity of periodontitis increased the percentage use of chlorhexidine increased while the use of mouth rinse with essential oils decreased. However, the study suggests the use of a mouth rinse is underutilized as a preventative therapy.
Patients should be considered in terms of health management and patient risk factors (such as plaque control and calculus) where they can take ownership of their oral condition. This person-centered approach requires that the patients have a diagnosis so they can understand how to manage their condition.23 The current system is not conducive for inclusion into the new healthcare paradigm and will be another lost opportunity for dentistry if change is not considered.
Clinical Implications
The study provides data for best practices related to revisions of procedure codes to diagnosis codes to integrate dentistry into the total healthcare system described by the electronic health record.
Conclusion
The practice of dentistry is presently limited in scope and opportunity as a procedure-based approach to patient treatment, where the CDT codes currently set the standard of care for treatment. The current system limits the approach of incorporating the philosophy of healthcare to health and considering the whole patient as a “person.”24 This article has provided data to suggest that the current system of procedure codes is not in the best interests of the patient or the dentist for diagnosis and treatment as well as referral. The system may need to be re-evaluated to be inclusive of the modern health paradigm as defined by the person/patient-centered medical home.25
Acknowledment
This study was supported by National Institute of Dental and Craniofacial Research (NIDCR) U01-DEO16755.
Disclosure
The authors report no conflicts of interest.
References
1. Health Insurance Portability and Accountability Act of 1996 (HIPAA). Pub. l. No. 104-191, 110 Stat. 1936 (1996).
2. Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9-21.
3. National Center for Health Statistics (NCHS) and the Centers for Medicare and Medicaid Services (CMS). International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed August 11, 2011.
4. Kalenderian E, Ramoni RL, White JM, et al. The development of a dental diagnostic terminology. J Dent Educ. 2011;75(1):68-76.
5. Spackman KA, Campbell KE, Cote RA. SNOMed RT: a reference terminology for health care. Proceedings of the American Medical Informatics Association. AMIA Fall Symposium. 1997:640-644.
6. Centers for Medicare and Medicaid Services. ICD-10. https://www.cms.gov/Medicare/Coding/ICD10/index.html. Accessed May 7, 2015.
7. Centers for Medicare and Medicaid Services. 2015 ICD-10-CM and GEMs. https://www.cms.gov/Medicare/Coding/ICD10/2015-ICD-10-CM-and-GEMs.html. Accessed April 24, 2015.
8. Martin JA, Grill AC, Matthews AG, et al. Periodontal diagnosis affected by variation in terminology. J Periodontol. 2013;84(5):606-613.
9. Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions [erratum, J Periodontol. 2003;74(10):1568.]. J Periodontol 2001;72(12):1790-1800.
10. Parameter on chronic periodontitis with advanced loss of periodontal support. American Academy of Periodontology. J Periodontol. 2000;71(5 suppl):856-858.
11. Parameter on chronic periodontitis with slight to moderate loss of periodontal support. American Academy of Periodontology. J Periodontol. 2000;71(5 suppl):853-855.
12. Sweeting LA, Davis K, Cobb CM. Periodontal Treatment Protocol (PTP) for the general dental practice. J Dent Hyg. 2008;82(3 suppl):16-26.
13. Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36(6):458-467.
14. Public Law 111-148. 111th United States Congress. Washington, DC: United States Government Printing Office; March 23, 2010. Retrieved 2013-12-22.
15. Curro FA, Vena D, Naftolin F, et al. The PBRN initiative: transforming new technologies to improve patient care. J Dent Res. 2012;91(7 suppl):S12-S20.
16. Curro FA, Thompson VP, Naftolin F, et al. Practice-based research network infrastructure design for institutional review board risk assessment and generalizability of clinical results. Therapeutic Innovation & Regulatory Science. 2013;47(1)82-89.
17. Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. 92(7):592-597.
18. Eke PI, Dye BA, Wei L. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-920.
19. Page RC, Eke, PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 suppl):1387-1399.
20. Veitz-Keenan A, Barna JA, Strober B, et al. Treatments for hypersensitive noncarious cervical lesions: a Practitioners Engaged in Applied Research and Learning (PEARL) Network randomized clinical effectiveness study. J Am Dent Assoc. 2013;144(5):495-506.
21. American Dental Association. CDT 2013. Current Dental Terminology: The ADA Practical Guide to Dental Procedure Codes. Chicago, IL: American Dental Association; 2012.
22. Curro FA. The clinical importance of drug evaluation. J Appl Res Clin Dent. 2005;2:10-17.
23. Robbins DA, Curro FA, Fox CH. Defining patient-centricity: opportunities, challenges, and implications for clinical care and research. Therapeutic Innovation & Regulatory Science. 2013:47(3):349-355.
24. Curro FA, Robbins DA, Millenson ML, et al. Person-centric clinical trials: an opportunity for the good clinical practice (GCP)-practice-based research network. J Clin Pharmacol. 53(10):1091-1094.
25. Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12):1202-1205.
About the Authors
Frederick A. Curro, DMD, PhD
Director
Practitioners Engaged in Applied Research and Learning (PEARL) Network
New York, New York
Ashley C. Grill, RDH, MPH
Coordinator, PEARL Network
New York, New York
Clinical Assistant Professor
Dental Hygiene Programs
New York University College of Dentistry
New York, New York
Abigail G. Matthews, PhD
Senior Biostatistician
EMMES Corporation
Rockville, Maryland
John Martin, DDS
Private Practitioner in Periodontics and PEARL Practitioner-Investigator
State College, Pennsylvania
Chief Science Officer
PreViser Corporation
Mount Vernon, Washington
Elisabeth Kalenderian, DDS, MPH, PhD
Chair
Oral Health Policy and Epidemiology
Chief of Quality
Harvard Dental Center
Harvard School of Dental Medicine
Boston, Massachusetts
Ronald G. Craig, DMD, PhD
Private Practitioner in Periodontics and Professor
Department of Basic Sciences and Craniofacial Biology
Department of Periodontology and Implant Dentistry
New York University College of Dentistry
New York, New York
Frederick Naftolin, MD, PhD
Professor of Obstetrics and Gynecology
Environmental Medicine Director
Reproductive Biology Research Co-Director
New York University Interdisciplinary Program in Menopause Medicine
Medical Director
PEARL Network
New York, New York
Van P. Thompson, DDS, PhD
Professor
Tissue Engineering and Biophotonics
King’s College London Dental Institute
Guy Hospital
London, England