Immediate and Durable Effects of an Oxalate Strip on Human Dentin In Vitro
Tiffany C. Hare, BS; Marianne Zsiska, PhD; Ying Boissy, BS; Tom E. Dufresne, PhD; Joseph H. Nurre, PhD; Paula A. Chmielewski, MS; and Phillip A. Drake, PhD
Abstract
Objective: The objective of this research was to evaluate the performance of a polyethylene strip coated with an oxalate-containing gel to occlude patent tubuli in human dentin.
Methods: An in vitro model was adapted from the published literature to create a physiologically relevant microenvironment to study immediate and long-term effects of the strip. Observation techniques included scanning electron microscopy (SEM), SEM of ion-milled surfaces (SEM/FIB), and synchrotron-based x-ray microtomography. In addition, the reduction in hydraulic conductance induced by the strip was quantified.
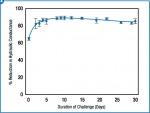
Results: Significant deposition of crystalline oxalate was observed in treated dentin. Crystal density and occlusionary performance were found to be strong functions of strip application time and of the number of applications. Quantitative reduction in hydraulic conductance correlated well with crystal accumulation, also demonstrating a strong dependence on time and number of applications. The robustness of an oxalate crystal barrier formed with a single 10-minute strip application was tested over a 30-day period, during which dentin samples were subjected to a series of dissolution and mechanical challenges. Oxalate crystal density was shown to be modestly impacted, with substantial flow resistance maintained throughout the 30-day challenge period.
Conclusion: The performance of an oxalate gel–coated strip is strongly affected by product–dentin contact time, producing an effective and durable occlusive barrier when applied as an adhesive strip.
The most broadly accepted mechanism for dentin hypersensitivity involves fluid movement through exposed tubuli.1-5 A pulse in pulpal fluid flow caused by a transient pressure gradient following thermal, tactile, or osmotic stimuli is thought to activate mechanoreceptors in Aδ fibers,6 which in turn causes a sharp pain sensation. Over-the-counter (OTC) therapies intended to directly interfere with nociception are based primarily on potassium salts. However, the onset of relief for potassium nitrate–based products appears to be slow, requiring weeks of ongoing use. In addition, results from recent controlled clinical trials have prompted questions regarding the magnitude of sensitivity relief possible with globally available potassium products.7-10
An alternative approach is to physically seal dentin tubuli against fluid movement. Products of this type are available in the OTC marketplace, but continuous daily use is required to maintain effectiveness. These products deposit superficial smear layers on the dentin surface, which vary significantly in durability, with most treatment modalities showing some level of susceptibility upon exposure to natural mechanical and dietary insult.11-14 Despite the progress in recent years to address dentin hypersensitivity with convenient at-home treatments, there is still an opportunity to provide more effective and durable OTC therapies.
Oxalates have been available as prescription products for antisensitivity for several decades. They have been shown in vitro to reduce hydraulic conductance to varying degrees by depositing oxalate crystals in and around lumina of tubuli.15-18 A wide variety of oxalate-containing formulations have been evaluated clinically. Clinical studies obtained with various oxalate salts have yielded variable results,19,20 particularly for short-duration, single-use products. Given the unique crystalline nature of oxalate deposits versus other occlusive chemistries, however, there is reason to expect that oxalate efficacy may be a strong function of the product delivery mode and/or formulation. This may be expected to be particularly true if the product form: (1) is spatially targeted to the sensitive area; and (2) provides protection from salivary dilution and mechanically disruptive processes during contact.
Considerable variation exists in the reported approach to in vitro evaluation of antisensitivity agents designed to occlude dentin tubuli. To mimic the physiological environment that exists during treatment with an occluding agent, several elements are likely important. These elements may include: (1) use of human dentin; (2) removal of unnatural smear layers introduced through the sectioning/cutting process necessary to obtain the dentin sample; (3) application of the test product according to labeled instructions, eg, use of a toothbrush for dentifrice, direct contact with strips, etc; (4) application of physiologically relevant fluid pressure, which produces counter-current liquid flow during the treatment cycle; (5) use of physiologically relevant “insult” pressure during measurement of initial and posttreatment hydraulic conductance (flow); and (6) application of a posttreatment chemical and/or mechanical challenge to the occlusive structure. It should be noted that the difficulty in placing a smear layer or crystalline plug may be quite different when opposed by an actively flowing liquid stream, as described in element number 4 above. This may be of special importance when evaluating therapeutic agents such as oxalate, which rely on diffusion to enter the lumen against the outward, convective current of pulpal fluid. Given the diameter of tubuli in human dentin, flow within these channels may be expected to exhibit a strong dependence on surface energy typical of true microfluidic systems. Therefore, a seventh important element may be the creation of a protein pellicle in the lumen and near the tubule orifice to create a realistic microenvironment.
Pioneering in vitro work by Brännström, Pashley, and others4,15-18 demonstrated the importance of hydraulic conductance (convective flow) as a measure of permeability as opposed to diffusion, and provided excellent examples of experimental apparatus to immobilize dentin sections and quantitatively evaluate the effect of therapeutic intervention on permeability. Reapplication of these models to address the above elements should provide a more accurate picture of the performance of an oxalate-based product.
Objective
The objective of this in vitro study was to evaluate the effectiveness of a novel antisensitivity strip coated with a 1.5% oxalate gel (Crest® Sensi-Stop™ Strips, Procter & Gamble, www.dentalcare.com) to occlude dentinal tubuli, with special attention given to the creation of a physiologically relevant microenvironment at the strip/tissue interface and within the lumen of the tubuli adjacent to the interface.
Materials and Method
Dentin Samples
Coronal dentin samples were prepared by sectioning maxillary and mandibular third molars parallel to the occlusal surface to obtain discs approximately 0.8-mm thick. The discs were obtained at an elevation to avoid both the appearance of pulpal horns on the cervical surface and any residual enamel on the coronal surface. The coronal surfaces of the samples were polished with 600-grit aluminum carbide paper before immersion in distilled water in an ultrasonic bath at 40 kHz for 6 minutes/side. Samples were then etched in a sonic bath for 2 minutes/side in 6% citric acid solution (pH 1.8), rinsed in distilled water, and stored in a commercial pH 7 phosphate buffer solution until use.
Mounting and Conditioning
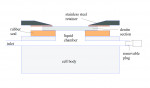
Samples were mounted in the split cell shown in Figure 1, which permitted the application of fluid pressure from the cervical side of the dentin section while providing access to the coronal surface for treatment without disassembly of the cell. This ensured continuity in the observation window defined by the nitrile rubber seal for quantitative measurements before and after treatment. A pellicle was formed on the lumen surfaces by flushing the dentin at 200 kPa for 15 minutes with Hartmann’s solution (30 mM lactic acid, 2 mM CaCl2, 5 mM KCl, 100 mM NaCl, adjusted to pH 7 with NaOH) to which 1.2% bovine serum albumin was added, after which the pressure was released and the coronal surface was immersed in whole saliva for 45 minutes. To stabilize the samples and prevent drift in hydraulic conductance, a power toothbrush (Oral-B® ProfessionalCare® SmartSeries 5000, Procter & Gamble) was applied to the coronal surface of the section while the section was subjected to 200 kPa of liquid flow of Hartmann’s solution. The initial flow was measured with a custom time versus position, bubble-based flowmeter to obtain a flow rate. The section was also subjected to a mechanical challenge consisting of manually brushing the coronal surface for 60 seconds using an American Dental Association (ADA) standard reference toothbrush with approximately 2 N of force to confirm flow stability. Two additional brushing/flow measurement cycles were completed and the flow was compared to the initial value. If postbrushing flow varied from the initial flow by < 5%, a baseline flow was calculated from the average of the three flow measurements. Specimens that exhibited unstable flow after multiple conditioning cycles were discarded.
Treatment Environment
The cell was flushed with Hartmann’s solution containing bovine serum albumin, and the pressure was reduced to a 30-cm head (3 kPa) before treatment of the dentin section. An arch punch was used to cut a circular disc from the oxalate-containing strip so that the disc diameter corresponded to the exposed coronal surface (9.5 mm). After excess solution was wicked from the dentin surface with a laboratory tissue, the circular cutout was placed gel side down on the surface for 10 minutes according to the product label, unless otherwise noted. The surface was then thoroughly rinsed with Hartmann’s solution to flush away the strip cutout and residual gel. The section was repressurized to 200 kPa and again brushed for 30 seconds before measurement of the posttreatment flow rate.
Durability/Substantivity
The durability of the induced flow reduction was evaluated over a 30-day period during which samples were repeatedly subjected to procedures intended to mimic some of the mechanical and chemical challenges typically experienced in the oral cavity. Procedures repeated daily for 5 days each week included: (1) mechanical brushing with 2 N of force with an ADA standard toothbrush for 60 seconds in Hartmann’s solution (morning); (2) mechanical brushing for 60 seconds in 10 mM citric acid at pH 2.5 (afternoon); and (3) a continuous rinse of Hartmann’s solution across the coronal surface and through the dentin under a head pressure of 30 cm (3 kPa). The liquid pressure was momentarily increased to 200 kPa after each citric acid challenge to obtain a flow rate measurement.
Sample Imaging
Samples were immersed in Hartmann’s solution during storage and transport to the imaging facility. Samples were prepared by air drying and then fracturing the dentin section by bending between separate forceps. Fractured samples were affixed to a mount positioned at 45°, and then coated with gold/palladium (Au/Pd) before scanning electron microscopy (SEM) imaging at 3 kV. Elemental mapping data was obtained with excitation at 10 kV. X-ray microtomography data were obtained with the assistance of Swiss Light Source’s TOMCAT beamline at the Paul Scherrer Institute in Villigen, Switzerland.
Results
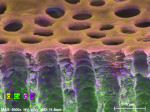
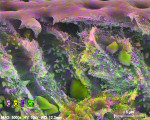
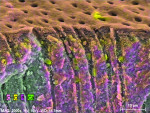
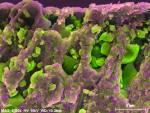
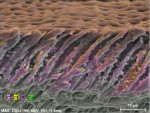
In contrast to untreated control samples (Figure 2), SEM images of tubuli in dentin that had been treated with the oxalate-containing strip were found to contain well-formed crystalline structures (Figure 3 through Figure 6). Oxalate crystals were located within the lumen, primarily between 5 µm and 25 µm from the coronal orifice. Under these experimental conditions, no crystals were found on the dentinal surface or deep within the lumen. Spatial mapping of the structures with energy dispersive x-ray spectroscopy (EDS) is consistent with the presence of an oxalate mineral in a hydroxyapatite-like mineral background.
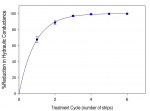
Crystal numerical density was found to be a strong function of treatment time (Figure 3 and Figure 4). Multiple application cycles also resulted in higher crystal densities (Figure 4 through Figure 6). After three applications of 10 minutes each, crystals appeared to occupy the whole of the lumen diameter.
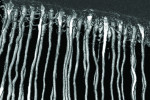
Although the spatial resolution afforded by x-ray tomography is lower than that of SEM, the high flux and narrow bandwidth of cyclotron radiation enables the acquisition of 3-dimensional (3D) data with sufficient clarity to visualize oxalate crystal deposition (Figure 7). To our knowledge, this is the first reported application of this valuable technique to image human tubuli. Examination of tomographic data (ie, virtual sections) obtained from treated samples confirmed that oxalate crystals are located primarily in the tubuli rather than on the surface of the treated dentin section. For samples treated with three strips, tomographic images perpendicular to the axis of the tubuli showed that the diameters of many crystals matched those of the tubule for complete occlusion against liquid flow. Figure 8 and Figure 9 show virtual slices of dentin at different depths, displaying crystalline occupation of the lumen in several tubuli after three treatment cycles. Continuous lengthwise examination of the lumen showed that complete occlusion was apparent at some point in each individual tubuli. No occlusion was observed with this technique in untreated samples (Figure 10).
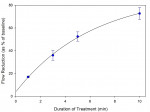
Quantitative hydraulic conductance data were also obtained to show the importance of treatment duration and the number of treatment cycles (Figure 11 and Figure 12). It was found that a single 10-minute treatment resulted in an average reduction in hydraulic conductance of approximately 70%. Complete cessation of fluid movement within the limitations of the method was typically obtained within 3 to 4 treatment cycles. Flow rates corresponding to as little as 0.05% of baseline flow were typically readily measurable in this model.
The durability of the occlusion afforded by treatment with a single strip was evaluated over the course of 30 days under conditions of regular brushing (10x/week) and continuous irrigation of dentinal tubuli with a simple pulpal fluid mimic (Hartmann’s solution) (Figure 13). Although a slight reversion in hydraulic conductance was observed after 10 days, the effect of treatment was still very much apparent after 30 days. Likewise, oxalate crystals in the tubuli of samples thus treated were observed via SEM to substantially endure to the end of the experiment, although a decrease was observed in the fraction of the lumen that appeared to be occupied (Figure 14).
Discussion
The predictive value of any in vitro model is a strong function of how closely it simulates the system it is designed to represent. To model product dissolution and oxalate release, a 30-cm head pressure (3 kPa) was applied to drive the flow of simulated pulpal fluid (bovine serum albumin–containing Hartmann’s solution) through the dentin section during product application. This was also felt to be important in realistically modeling the diffusion of oxalate into the lumen, which is opposed by and strongly affected by outward convective flow. In addition to the use of simulated pulpal fluid, specimens were incubated in human saliva before product application to simulate the natural environment on and around the dentin surface. Crystal density, morphology, and depth—all which may be argued as critical to efficacy and substantivity—are likely each a strong function of fluid composition, flow rate, and the surface on which deposition occurs.
The pressure that drives pulpal fluid at rates sufficient to stimulate pain is by definition higher than resting pulpal pressure, and may indeed be higher by several orders of magnitude.5 Although Poiseuille’s law states that flow should be a linear function of applied pressure, deviations from linearity have been noted in dentinal systems.21 To conservatively evaluate the effect of treatment with the oxalate strip, applied pressure was increased to 200 kPa for all measurements of hydraulic conductance. This value is consistent with pressure gradients reported to produce moderate levels of sensitivity pain in vivo.5
Qualitative (Figure 3 through Figure 5) and quantitative data (Figure 11 through Figure 13) obtained under these experimental conditions indicate the potential value of the 10-minute treatment time afforded by an adhesive oxalate strip. Targeted delivery of a relatively small quantity of oxalate gel to sites in the oral cavity in which dentin is exposed, and protection against salivary dilution or removal by mechanical action during treatment, enables a robust deposition of oxalate crystals inside dentinal tubuli. The oxalate-based deposition produced by treatment with a single strip was still evident after 30 days of daily dissolution and mechanical challenge, demonstrating the durability of the occlusive layer.
Importantly, the predictive value of the in vitro model was confirmed by clinical data. Amini and colleagues demonstrated a significant desensitizing benefit immediately after application of the oxalate strip with both air and tactile stimuli.22 Further, they found that the benefit was still evident 30 days after application of the last strip.
Conclusion
Deposition of crystalline oxalate from an oxalate gel–coated strip was shown to be strongly affected by conditions of use. Product application time was shown to be particularly important, with a substantial reduction in hydraulic conductance observed at 10 minutes versus 1 minute. Although multiple treatment cycles provided complete occlusion, a single treatment reduced flow by approximately 70%, which largely endured continuous dissolution and mechanical challenges over a 30-day period. The model demonstrates that this innovative and convenient antisensitivity treatment has the potential to provide substantial, immediate, and long-lasting relief to sensitivity sufferers.
Disclosure
This research was sponsored by Procter & Gamble.
About the Authors
Tiffany C. Hare, BS
Principal Researcher
Procter & Gamble
Mason, Ohio
Marianne Zsiska, PhD
Senior Scientist
Procter & Gamble
Mason, Ohio
Ying Boissy, BS
Principal Researcher
Procter & Gamble
Cincinnati, Ohio
Tom E. Dufresne, PhD
Principal Scientist
Procter & Gamble
Mason, Ohio
Joseph H. Nurre, PhD
Senior Scientist
Procter & Gamble
Mason, Ohio
Paula A. Chmielewski, MS
Senior Scientist
Procter & Gamble
Mason, Ohio
Phillip A. Drake, PhD
Senior Scientist
Procter & Gamble
Mason, Ohio
References
1. Gysi A. An attempt to explain the sensitiveness of dentin. Br J Dent Sci. 1900;43:865-868.
2. Brännström M. A hydrodynamic mechanism in the transmission of pain producing stimuli through the dentin. In: Anderson DJ, ed. Sensory Mechanisms in Dentine. Oxford: Pergamon Press; 1963:73-79.
3. Brännström M, Johnson G, Linden LA. Fluid flow and pain response in the dentine produced by hydrostatic pressure. Odontol Revy. 1969;20(1):15-30.
4. Brännström M, Linden LA, Aström A. The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res. 1967;1(4):310-317.
5. Ahlquist M, Franzén O, Coffey J, Pashley D. Dental pain evoked by hydrostatic pressures applied to exposed dentin in man: a test of the hydrodynamic theory of dentin sensitivity. J Endod. 1994;20(3):130-134.
6. Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35(4):300-315.
7. West NX, Addy M, Jackson RJ, Ridge DB. Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. J Clin Periodontol. 1997;24(4):209-215.
8. Poulsen S, Errboe M, Hovgaard O, Worthington HW. Potassium nitrate toothpaste for dentine hypersensitivity. Cochrane Database Syst Rev. 2001;(2):CD001476.
9. Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;(3):CD001476.
10. Karim BF, Gillam DG. The efficacy of strontium and potassium toothpastes in treating dentine hypersensitivity: a systematic review. Int J Dent. 2013;doi:10.1155/2013/573258.
11. Sharma D, Hong CX, Heipp PS. A novel potassium oxalate-containing tooth-desensitising mouthrinse: a comparative in vitro study. J Dent. 2013;41(suppl 4):S18-S27.
12. Parkinson CR, Willson RJ. A comparative in vitro study investigating the occlusion and mineralization properties of commercial toothpastes in a four-day dentin disc model. J Clin Dent. 2011;22(3):74-81.
13. Zsiska M, White DJ, Moore JA. Acid durability of rapid-onset smear layers produced by dentifrices. Paper presented at: 89th General IADR Session; March 16-19, 2011; San Diego, CA.
14. West NX. The dentine hypersensitivity patient-a total management package. Int Dent J. 2007;57(S6):411-419.
15. Greenhill JD, Pashley DH. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res. 1981;60(3):686-698.
16. Pashley DH, O’Meara J, Kepler EE, et al. Dentin permeability. Effects of desensitizing dentifrices in vitro. J Periodontol. 1984;55(9):522-525.
17. Pashley D, Galloway S. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol. 1985;30:731-737.
18. Pashley DH. Dentin permeability, dentin sensitivity and treatment through tubule occlusion. J Endod. 1986;12(10):465-474.
19. Cunha-Cruz J, Stout JR, Heaton LJ, Wataha JC. Dentin hypersensitivity and oxalates: a systematic review. J Dent Res. 2011;90(3):304-310.
20. Sharma D, McGuire JA, Amini P. Randomized trial of the clinical efficacy of a potassium oxalate-containing mouthrinse in rapid relief of dentin sensitivity. J Clin Dent. 2013;24(2):62-67.
21. Camps J, Giustiniani S, Dejou J, Franquin JC. Low versus high pressure for in vitro determination of hydraulic conductance of human dentine. Arch Oral Biol. 1997;42(4):293-298.
22. Amini P, Miner M, Sagel PA, Gerlach RW. Comparative effects of 1.5% oxalate strips versus 5% potassium nitrate dentifrice on dentin hypersensitivity. Compend Contin Educ Dent. 2016;37(spec iss 1):21-25.