Do Bib Clips Pose a Cross-Contamination Risk at the Dental Clinic?
Abstract:
Although multiple-use dental napkin holders have a relatively low risk of transmitting infection, they do require disinfection between patients. This study sought to: 1) determine the presence of bacterial load on two types of clips of reusable bib chains after dental procedures at the Endodontics and Orthodontics clinics at Tufts University School of Dental Medicine; and 2) evaluate the effectiveness of disinfecting the clips. These specialty clinics represent a wide spectrum of patients, procedures, and appointment times. Bacterial load on the bib clips was determined immediately following dental treatments—both before and after their disinfection—during morning and afternoon sessions. The results revealed that, after treatments, there was a statistically significant difference when comparing the two clinics for bacterial burden on the clips. Furthermore, there was a statistically significant difference in bacterial load on the two types of clips. Disinfection of the bib clips was highly effective in both clinics. Clinically, the results suggest that due to the nature of the treatment and the type of bib clips used, patients in different clinics may be exposed to varying bacterial concentrations on the bib clips, and thus to different possible cross-contamination risks. Future analyses will be performed to identify the bacterial species in samples from both pre- and post-disinfected clips, and to determine if they harbor disease-causing bacterial species that can pose a potential, yet undetermined risk for cross-contamination.
Dental instruments are either disposable or meant for multiple patient uses. Multiple-use items include dental mirrors, ultrasonic units, and patient napkin holders (ie, bib chains). These items require sterilization or disinfection procedures to reduce the risk of transmitting infection. Items contacting intact skin only, such as bib chains, have a relatively low risk of transmitting infection, and as such may be subject to Centers for Disease Control and Prevention (CDC) regulations requiring the use of intermediate- or low-level disinfectants.1 A previous study by Molinari demonstrated that bib chains harbored bacteria on their surface following dental treatments.2 Furthermore, that study found that a brief wipe of the chains with an EPA-approved, intermediate-level disinfectant between patient appointments reduced, but did not eliminate, the microbial load found on these chains.2 Similarly, the presence of bacterial contamination on bib clips after treatment and after disinfection was also found in a study performed at the Dental School of Witten/Herdecke University in the Witten area in Germany.3 However, no published studies document the bacterial loads on bib clips following different dental treatments in specialty clinics, such as Endodontics and Orthodontics, and the effectiveness of disinfection of bib clips after patient care.
Study Objectives
The primary objectives of this study were as follows: 1) to determine the presence of bacterial contaminants on two types of clips of reusable patient bib chains following dental procedures at Endodontics and Orthodontics clinics; and 2) to evaluate the effectiveness of disinfection of these clips using a commercially available disinfectant.
Materials and Methods
Bib Clips and Tested Parameters
Two types of bib clips were used in this study—metal clips that are attached to metal bib chains (Henry Schein napkin holder, Henry Schein, Inc., www.henryschein.com) and rubber-faced metal clips that are attached to rubber bib chains (Slip-Nots™, Kerr TotalCare, Metrex Research Corp., www.metrex.com). Bib clips were sampled during the morning (AM) and afternoon (PM) sessions at the Endodontics and Orthodontics department clinics at Tufts University School of Dental Medicine (TUSDM). In each department, a total of 40 chains were studied; among them, 20 had metal clips and 20 had rubber-faced metal clips. From each chain, only the clips were sampled for bacterial contamination. The study was conducted according to an approved protocol by Tufts Medical Center and Tufts University Health Sciences Institutional Review Board (IRB) office.
Bacterial Sample Collection
Bib clips were sampled for bacterial contaminants immediately following dental treatments provided to patients. Sampling was done both before and after the clips were disinfected by wiping them with CaviCide® wipes (CaviWipes®, Metrex Research Corp.) and letting them dry according to the manufacturer’s instructions and the disinfection protocol followed at TUSDM. CaviCide active ingredients are 17.2% isopropanol and 0.28% diisobutylphenoxyethoxyethyl dimethyl benzyl ammonium chloride. Immediately after patient dismissal, bacterial contaminants were directly transferred from the clips onto Trypticase soy agar plates in the operatory. A direct transfer was achieved by firmly pressing each of the four sides of each clip onto a separate area on a nutrient agar plate.4 Thereafter, the clips were disinfected by wiping them with CaviCide and sampled again in the same manner for bacterial contaminants on a separate agar plate. Plates were then transferred to a microbiology laboratory at TUSDM and incubated aerobically at 37°C for 48 hours. Bacterial samples were collected from both the right and left clips of each chain, and the total number of bacterial colonies developed from inoculation was considered to be one sample. For each plate, the number of bacterial colonies that grew on the agar was recorded, and representative plates were imaged.
Statistical Power
A power calculation was determined using NQuery Advisor 7.0 (Statistical Solutions, www.statistical-solutions-software.com). Assuming an effect size of δ = 1.289 (based on the results of a pilot study), a sample size of n = 80 (of which 40 chains had metal clips, and 40 chains had rubber-faced metal clips) was considered adequate to achieve a Type I error rate of α = 0.05 and a power over 99%.
Statistical Analysis
Descriptive statistics tabulated included means, medians, standard deviations, lower and upper quartiles, and minima and maxima. Because the data exhibited substantial non-normality, a non-parametric hypothesis testing was conducted via the Mann-Whitney U test. P values less than 0.05 were considered statistically significant. All analyses were completed using SAS®, version 9.2 (SAS Institute Inc., www.sas.com).
Results
Bacterial Load on Pre-Cleaned Bib Clips
As expected, the study demonstrated that following a dental treatment, bacterial contaminants persisted on pre-cleaned bib clips. Furthermore, the results indicated a clear difference in bib clips’ contamination levels between the two departments participating in this study, and also between the two types of clips that were tested (Table 1).
From the Endodontics clinic, a total of 1,239 bacterial colonies grew on the agar plates, of which 389 colonies developed from metal clip samples, and 850 colonies developed from samples of rubber-faced metal clips (Table 1, A). An average of 19.5 and 42.5 bacterial isolates grew from the metal clips and the rubber-faced metal clips, respectively, per sampled bib chain (Table 1, B). In addition, per metal chain, an average of 27.4 and 16.8 bacterial isolates grew from the clips sampled during the morning and afternoon sessions, respectively (Table 1, C). These numbers increased to an average of 40 and 44.2 bacterial isolates per chain with rubber-faced metal clips sampled during the morning and afternoon sessions, respectively (Table 1, C).
In contrast to these findings, from the Orthodontics clinic, a total of 3,563 bacterial colonies developed on the agar plates, of which 970 colonies developed from metal clip samples, and 2,593 bacterial colonies developed from samples of rubber-faced metal clips (Table 1, A). Table 1 shows that per bib chain sampled in this department, an average of 48.5 and 129.7 bacterial isolates developed from samples of metal clips and rubber-faced metal clips, respectively (Table 1, B). An average of 27.7 and 59.7 bacterial isolates grew from the metal clips of each chain sampled during the morning and the afternoon sessions (Table 1, C). These numbers increased to an average of 104.7 and 154.6 bacterial isolates per chain, sampled from rubber-faced metal clips taken during the morning and afternoon sessions (Table 1, C).
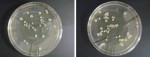
Representative images of nutrient agar plates with bacterial growth from clips sampled following dental treatment are shown in Figure 1. Taken together, when stratified by departments, bacterial counts following dental treatment revealed a three-fold increase in the total bacterial amount found on sampled bib clips in the Orthodontics clinic in comparison to the Endodontics clinic. When stratified by clip type, the authors’ findings indicated a two-fold and a 2.5-fold increase in the number of bacterial contaminants on metal clips when compared to rubber-faced metal clips in the Endodontics and Orthodontics clinics, respectively. The descriptive statistics of bacterial counts, stratified by pre-cleaning and post-cleaning, and then by department, time of day, and type of clip are shown in Table 2. The data demonstrate that following dental treatments, there was a statistically significant difference when comparing both departments in the pre-cleaned sets (P = 0.002) (Table 2). Furthermore, there was a statistically significant difference between the two types of clips (P = 0.0001). However, with a P value of 0.750, the study could not demonstrate a statistically significant difference between clip samples that were collected in the morning session as opposed to those that were collected in the afternoon session (Table 2, A).
Bacterial Load on Post-Cleaned, Disinfected Bib Clips
After disinfection of bib chains, comparable results were obtained when clips were sampled in both departments, as shown in Table 1. A total of 23 bacterial colonies developed from samples collected from the Endodontics clinic, 10 of which developed from metal clips, and 13 from rubber-faced metal clips (Table 1, A). Similarly, from the Orthodontics clinic, 21 colonies developed on the agar, 9 of which developed from metal clip samples, and 12 from samples of rubber-faced metal clips (Table 1, A). Representative images of nutrient agar plates with bacterial growth from clips sampled following their disinfection are shown in Figure 2. After disinfection, in both departments, with the total number of sampled chains taken into account, an average of 0.45 to 0.65 bacteria per chain was found with either clip types (Table 1, B). In addition, Table 1, C indicates that when compared to the morning sessions, an average of one or more bacterial isolates developed from rubber-faced metal clips sampled in the afternoon session in both clinics. The data from the statistical tests for the post-cleaned samples did not demonstrate a significant difference when comparing bacterial counts between departments (P = 0.671), time of day (P = 0.068), and type of clip (P = 0.791) (Table 2, B). With significance set at P = 0.05, the P value for the comparison of counts between the morning and afternoon sessions could be considered approaching significance with a value of 0.068. Thus, after disinfection, persistence of bacterial contaminants on bib clips may occur as the day at the clinic progresses.
Finally, the results in Table 1, D indicate that of the total number of tested chains, one or more bacterial colonies developed after sampling the metal clips of six bib chains and the rubber-faced metal clips of five bib chains in the Endodontics clinic. Similarly, one or more bacterial colonies grew after sampling the metal clips of five bib chains and the rubber-faced metal clips of four bib chains in the Orthodontics clinic. Clinically, and similarly to previous studies2,3,5, these data show that remaining bacteria were detected on 20% to 30% of the bib clips following disinfection (Table 1, D).
Discussion
This study sought to determine the presence of bacterial contaminants on bib clips and the effectiveness of disinfecting these clips. Both the Endodontics and the Orthodontics specialty clinics participated in this study, representing a wide spectrum of patients, dental procedures, and appointment times. The procedures performed in the Endodontics clinic included consultation, nonsurgical endodontic therapy, and orthograde retreatment. Procedures performed in the Orthodontics clinic included consultation, impressions, bracket and band placement, appliance adjustment, orthodontic wire adjustments, as well as braces removal, polishing, and debriding.
Similar to the findings reported by Molinari,2 bib clips sampled in both clinics exhibited bacterial contamination immediately after dental procedures. Molinari’s study, however, does not identify the types of clinics or treatments performed. Following treatment and before disinfection, the present study found a greater microbial load on bib clip samples from the Orthodontics clinic when compared to samples from the Endodontics clinic. This finding raises the question as to why a higher level of bacterial load is found in the orthodontic setting as opposed to the endodontic setting. The statistically significant difference in bacterial contamination found on bib clips following treatment is likely due to the nature of the treatments provided to patients in these clinics. It may result primarily from the use of rubber dams during an endodontic treatment, which limits salivary contamination of the bib clips. In orthodontics, a significant aspect of the treatment involves entering and re-entering the oral cavity with appliances, thus bacterial contamination from the gloves could be transferred to the patient’s bib clips. If the rubber dam in the endodontics setting reduces the bacterial load, it must be understood that the use of a rubber dam in an orthodontics setting is actually impractical. In addition, this difference may also result from the application and adjustment of the clips by the patients themselves in the Orthodontics clinic. Collectively, the study’s findings suggest that due to the nature of the dental treatment, different clinics may be exposed to different bacterial loads and bacterial species on the clips, and, therefore, to a different contamination risk.
The present study also revealed a significant and clinically important difference in bacterial loads between the two types of bib clips that were sampled following treatments. In comparison to the metal bib clips, the rubber-faced metal clips demonstrated a significantly greater number of bacterial contaminants. One possible explanation is that due to its nature, the rubber material may exhibit microscopic porosities, which can support bacterial adherence and, in general, may decrease disinfection efficiency. In contrast, the smooth surface of metal bib clips may reduce bacterial adherence and, in general, their disinfection may be more effective.
Molinari’s study suggested that bacterial loads on bib chains can occur due to extended contact of the bib chain with the patient’s skin, exposure of the chain to microbe-containing aerosols during treatment, and handling the chains with contaminated gloves during the treatment.2 By sampling only the bib clips, the present study excluded bacterial contaminants that can be found on the rest of the chain, specifically the areas that are in close contact with the patient’s skin. Thus, its findings represent the actual bacterial load that resides on the bib clips due to the dental treatment and/or the surrounding environment—ie, airborne bacteria—after dental treatment and after disinfection. However, immediately after treatment, skin commensals are likely to be found on the bib clips from young patients adjusting the bib chain in the Orthodontics clinic.
In addition, while no significant difference was found between the clinics or the type of clip in the post-cleaned samples, the P value for the comparison of bacterial counts obtained in the morning and afternoon sessions could be considered approaching significance. Clinically, these results suggest that bacteria may be accumulating on the bib clips between the morning and the evening sessions, as patients are treated throughout the day.
Finally, the findings of the present study demonstrate that despite the initial, marked difference in bacterial load on pre-cleaned bib clips in both clinics and on both clip types, effective bacterial reduction is achieved by the disinfection protocol. However, whereas 70% to 80% of the clips were left with no detectable bacteria after disinfection, 20% to 30% of the disinfected clips still harbored bacterial contaminants on their surfaces that may or may not pose a cross-contamination risk during patient care at the dental clinic. These results are similar to those recently found in a study performed by the University of North Carolina at Chapel Hill’s School of Dentistry.5 In the current study, the mere presence of bacterial contaminants on the disinfected clips has not been shown to be an infectious disease problem. However, the identity of the bacteria found on the pre- and post-disinfected clips remains unknown. To address cross-contamination issues by bib clips in patient care in different clinics, further investigation should be performed to determine the species and strains of the bacterial contaminants present on the bib clips after dental treatment and following disinfection.
Conclusions
Results of this study indicated that following dental procedures, a significantly greater bacterial burden is present on bib clips in the Orthodontics clinic as compared to those in the Endodontics clinic. Furthermore, when metal clips are compared to clips with rubber facings, greater numbers of bacteria were found on the latter. While disinfecting the clips was proven to be significantly effective, 20% to 30% of both clip types still harbored bacteria, posing a potential yet undetermined risk for cross-contamination.
This novel study design, which was implemented in specialty clinics, establishes baseline data for further analyses in additional clinics. Moreover, further research should be performed in order to determine the identity of bacterial species in samples from both pre- and post-disinfected bib clips and to determine whether or not they include disease-causing bacterial species.
Acknowledgments
The authors wish to thank Dr. Daniel B. Green, Chair of the Endodontics Department, and Dr. Barry S. Briss, Chair of the Orthodontics Department at TUSDM for their stimulating discussions and assistance in this study. Special thanks also to Dr. James B. Hanley, and Maureen Lombard from the Department of Clinical Affairs at TUSDM for their helpful comments and suggestions regarding this study.
Disclosure
The authors have no conflict of interest. The study was sponsored in part by Dux Dental (www.duxdental.com).
References
1. Centers for Disease Control and Prevention. Guidelines for infection control in dental health care settings. MMWR. 2003;52(RR-17):1-61.
2. Molinari JA. Microbial contamination of patient napkin holders. The Dental Advisor. June 2010, Number 29.
3. German Study Finds Bacteria on 70% of Dental Bib Holders [press release]. S. Zimmer, University of Witten/Herdecke in Witten, North Rhine-Westphalia, Germany. Dr Bicuspid Hygiene Community; March 19, 2012.
4. Alt-Holland A, Thondapu RS, Rajapaksha KS, et al. A novel method to examine bacterial contamination in dental clinics [abstract]. J Dent Res. 2012;91(spec iss A). Abstract 659.
5. Study Finds Bib Chain Potential Source of Bacteria [press release]. Oral Microbiology Laboratory, Chapel Hill’s School of Dentistry, University of North Carolina. Dental Health Magazine; March 16, 2010.
About the Authors
Addy Alt-Holland, BSc, MSc, PhD
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Sreedevi Srinivasan, BDS, DSc
Department of Orthodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Rebekah Lucier, DMD
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Claire L. Kublin, BS
Department of Research Administration
Tufts University School of Dental Medicine
Boston, Massachusetts
Jennifer M. Fong, DDS
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Joshua Goldfein, DMD
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
David L. Baker, DMD
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Angel Park, MS, MPH
Department of Research Administration
Tufts University School of Dental Medicine
Boston, Massachusetts
Matthew Finkelman, PhD
Department of Research Administration
Tufts University School of Dental Medicine
Boston, Massachusetts
Toshihisa Kawai, DDS, PhD
Department of Immunology and Infectious Diseases
The Forsyth Institute
Cambridge, Massachusetts
Department of Oral Medicine
Infection & Immunity
Harvard School of Dental Medicine
Boston, Massachusetts
Bruce J. Paster, PhD
Department of Microbial Ecology and Pathogenesis
The Forsyth Institute,
Cambridge, Massachusetts
Department of Oral Medicine,
Infection & Immunity
Harvard School of Dental Medicine
Boston, Massachusetts
Gerard Kugel, DMD, PhD
Department of Research Administration,
Tufts University School of Dental Medicine,
Boston, Massachusetts