Cell Adhesion to Acrylic Custom Provisional Abutment Placed on an Immediate Implant: A Case Report
Hanae Saito, DDS, MS, CCRC; Ru-Ching Hsia, PhD; Dennis P. Tarnow, DDS; and Mark A. Reynolds, DDS, PhD
ABSTRACT
This article presents the results of a scanning electron microscope (SEM) analysis of the surface of an acrylic custom provisional abutment following first disconnection from a post-extraction immediate implant placement. An implant was placed immediately after extraction, the site was grafted, and a barrier membrane was adapted for graft containment. A custom acrylic shell was then relined, polished, and steam-cleaned prior to being screwed onto the implant. After 5 months of undisturbed healing, the custom provisional abutment was disconnected for the first time and processed for SEM examination. The surface of the custom acrylic abutment revealed well-spread fibroblast-like cells with filopodia inserting into the porous surface. These observations suggest that the surface topography of the acrylic provisional restoration/ abutment can function as a substratum for cellular adhesion and may serve an important role in supporting peri-implant mucosa at the time of immediate implant placement.
Immediate implant placement with a provisional restoration in nonfunctional occlusion in the maxillary anterior esthetic region has gained increasing clinical adoption.1 Chu et al2 recently reported that immediate implant placement with a provisional restoration helped maintain the vertical and bucco-lingual dimensions of midfacial soft tissue at the time of anterior tooth extraction. Presumably, the provisional restoration provided mechanical support of the peri-implant mucosa, including the marginal and papillary tissues. The provisional restoration may also help contain and stabilize the bone-graft material during healing.3
In a previous clinical study, bleeding of the peri-implant mucosa upon first disconnection of the provisional restoration was associated with significantly less dimensional change of the bucco-lingual ridge than sites without bleeding on removal of the provisional restoration.4 In the latter study, the provisional restoration was contoured to mirror the cervical morphology of the extracted tooth, polished, and steam cleaned prior to insertion. The custom provisional restoration and abutment appeared to provide a platform for peri-implant mucosal healing, thereby minimizing the change in bucco-lingual dimension. In the presence of adhesion of the peri-implant mucosa to the acrylic, sulcular bleeding upon removal of the abutment and provisional restoration would be consistent with disruption of this interface.5
One potential role of the provisional restoration/abutment is to provide a surface for adhesion of the peri-implant soft tissue during the early stages of healing. Multiple cell types, including fibroblasts and epithelial cells, and extracellular matrix components can adhere to dental materials, such as titanium and gold, when those materials are placed on natural teeth.5,6 When properly contoured and polished, acrylic restorations appear to support gingival form; moreover, epithelial cells have been shown to adhere to the surface of acrylic crowns placed below the gingival margin.7
The role of the provisional restoration/abutment in wound healing at the time of flapless post-extraction immediate implant placement remains incompletely understood. Recent evidence suggests that the provisional restoration/abutment may serve an important function in supporting adhesion of peri-implant mucosa at the time of immediate implant placement. The purpose of this scanning electron microscope (SEM) analysis, therefore, was to microscopically examine the surface of an acrylic custom provisional abutment following first disconnection from a post-extraction immediate implant.
Case Report
Clinical Procedure
An 87-year-old Asian man presented for treatment of the maxillary left first premolar following failure of endodontic therapy. Upon examination, the tooth exhibited a 9-mm probing depth on the buccal aspect. All findings, treatment options, and risks were reviewed with the patient, who consented to extraction, grafting, and immediate implant placement and provisional restoration.
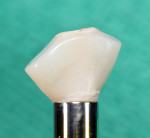
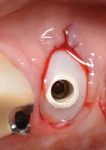
The roots were sectioned and extracted without flap elevation. After debridement, the extraction socket was classified as a type II8; therefore, a resorbable collagen barrier membrane and particulate inorganic bovine bone graft (BioOss™ Geistlich Pharma North America, Inc., geistlich-na.com) were placed into the gap. A threaded, textured-surface implant was placed to a depth of 3 mm from the free gingival margin. A screw-retained custom provisional abutment was fabricated as a single unit using a preformed acrylic shell filled with autopolymerizing resin (Super T, American Consolidated Manufacturing Co.) and a polyether-ether-ketone (PEEK) polymer abutment9 (Figure 1). The provisional abutment was designed to capture the subgingival profile of the peri-implant soft tissue.10 Prior to insertion of the custom provisional abutment, the subgingival surface was steam cleaned for 20 seconds (TouchSteam™, Kerr Dental, kerrdental.com) and the abutment secured with a screw (Figure 2). The patient was given a prescription for a postsurgical broad-spectrum antibiotic and analgesic medication, as needed, and was seen for follow-up 14 days postoperatively.
After 5 months of undisturbed healing, the custom provisional abutment was removed for the first time since implant placement to make an impression. The patient demonstrated evidence of good oral hygiene (eg, plaque-free score higher than 80%). The treating surgeon obtained verbal and written informed consent based on the Helsinki Declaration to permit SEM analysis of the removed custom provisional abutment and subsequent publication of clinical and SEM findings. The patient received the final restoration 6.5 months after surgical implant placement.
Specimen Preparation and Imaging
The removed custom provisional abutment was immediately stored in 10% formaldehyde solution and processed for SEM analysis. The acrylic specimen was air-dried in a hood overnight, mounted on SEM pin mounts, and sputter coated with 10 nm to 20 nm of platinum/palladium in a sputter coater (EMS 150T ES, Electron Microscopy Sciences, emsdiasum.com). SEM images were taken in a scanning electron microscope (Quanta 200, FEI, fei.com) under the conditions specified in the image.
Immunogold Cell Labeling
For SEM imaging, the acrylic provisional abutment was quenched with 50 mM glycine in 0.1 M phosphate buffer for 15 minutes. After washing three times with 0.1 M phosphate buffer for 30 minutes, the abutment was incubated with rabbit anti-fibroblast specific protein (S100A4, EMD Millipore, emdmillipore.com), washed three times in rinse buffer, and incubated with goat anti-rabbit immunoglobulin G conjugated with nanogold (Nanoprobes, Inc., nanoprobes.com). The abutment was then washed with buffer three times and fixed with 2% glutaraldehyde. Nanogold particles bound to the specimen were enlarged by gold enhancement (Nanoprobes, Inc.) following the manufacturer’s recommendation.
The abutment was post-fixed with 1% osmium tetroxide in water, washed again in 3 changes of water, and dehydrated in serial-graded ethyl alcohol. Lastly, the abutment was chemically dried by immersing sequentially in hexamethyldisilazane (Electron Microscopy Sciences) and air-dried overnight. Specimens were mounted on SEM pin mounts and carbon coated in a carbon evaporator (EMS 150T ES).
Clinical Observations
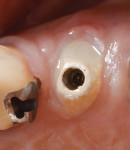
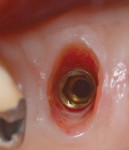
Bleeding from the peri-implant sulcus was clinically evident at the time of disconnection of the acrylic provisional abutment and provisional restoration (Figure 3). The implant site did not exhibit clinical signs of inflammation before the disconnection of the custom provisional abutment; the patient exhibited a Silness-Löe plaque index of 15%.11 No discernable change in bucco-lingual ridge dimension was noted at 5 months (time of impression) and 12 months after the implant placement (Figure 4 and Figure 5).
SEM Observations
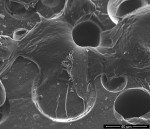
SEM imaging was focused on the surface of the custom provisional abutment placed in the peri-implant sulcus. The SEM images revealed well-spread fibroblast-like cells with filopodial extensions inserting into the porous surface of the custom provisional abutment (Figure 6 and Figure 7). Immunogold labeling expressing fibroblast-specific protein-1 confirmed the presence of cells under backscattered microscope in a corresponding area (Figure 8 and Figure 9). Areas of acrylic surface devoid of cells were also found. Similarly, areas of the acrylic surface, particularly toward the marginal peri-implant mucosa, exhibited evidence of bacterial biofilm formation.
Discussion
Bleeding of the peri-implant mucosa at the time of first disconnection of acrylic provisional abutment/restoration following immediate implant provisionalization has been associated with dimensional stability (bucco-lingual) of the ridge.4 This observation suggests that the provisional abutment/restoration may serve a role in supporting adhesion of peri-implant mucosa at the time of immediate implant placement, and, perhaps, in providing a more effective biologic barrier. Nevertheless, sulcular bleeding could reflect ulceration of the peri-implant “sulcular” mucosa, disruption of an adherent mucosa–acrylic interface, or both. The SEM images in this case revealed well-spread fibroblast-like cells with filopodia inserting into the porous surface of the custom provisional abutment. Although no clinical signs of inflammation were evident, areas of the acrylic surface exhibited evidence of bacterial biofilm formation, particularly toward the marginal peri-implant mucosa; such areas were devoid of cells and, presumably, associated with mucosal inflammation.12,13
Prior studies have examined the clinical application of resin materials and the effects on soft tissue. Using a light microscope, Waerhaug and Zander14 showed that epithelium can adhere to a clean surface of artificial crowns, and this concept was applied to the present study. Listgarten and Lai15 demonstrated the preservation of an intact epoxy-resin–made implant and soft-tissue interface. In the present study, the subgingival surface of the fabricated screw-retained custom provisional abutment was filled with autopolymerizing resin, and the PEEK abutment was steam cleaned for 20 seconds after being polished before being placed on the implant.
Surface topography has been shown to affect gingival fibroblast attachment, orientation, and migration.16-18 For example, Chehroudi et al19 reported that 19-μm- and 30-μm-deep grooves or 120-μm-deep tapered pits on the surface of titanium-coated implants encouraged connective tissue ingrowth, and Kim et al20 observed oblique fibroblast orientation on surfaces with 30-μm grooves. In the present study, regions of the acrylic abutment exhibited well-spread fibroblast-like cells with filopodia inserting into the porous surface. Presumably, adhesion of peri-implant mucosa following immediate implant placement would both impede epithelial downgrowth and create a more effective biologic barrier. Wound stabilization should promote greater stability of the alveolar ridge.16,21
Conclusion
In this case report, an acrylic provisional restoration/abutment exhibited regions with cellular adherence and spreading, often in association with surface microporosities. This observation suggests that the acrylic surface can function as a substratum for cellular adhesion and may serve an important role in supporting peri-implant mucosa at the time of immediate implant placement.
ACKNOWLEDGMENT
The authors would like to thank Adam J. Mieleszko, CDT, for his laboratory support in the fabrication of the custom provisional abutment and the discs.
ABOUT THE AUTHORS
Hanae Saito, DDS, MS, CCRC
Assistant Professor, Division of Periodontics, University of Maryland School of Dentistry, Baltimore, Maryland
Ru-Ching Hsia, PhD
Associate Professor, Core Imaging Facility, University of Maryland School of Dentistry, Baltimore, Maryland
Dennis P. Tarnow, DDS
Clinical Professor, Director of Implant Education, Columbia University College of Dental Medicine, New York, New York
Mark A. Reynolds, DDS, PhD
Professor, Division of Periodontics, University of Maryland School of Dentistry, Baltimore, Maryland
REFERENCES
1. Wöhrle PS. Single-tooth replacement in the aesthetic zone with immediate provisionalization: fourteen consecutive case reports. Pract Periodontics Aesthet Dent. 1998;10(9):1107-1114.
2. Chu SJ, Salama MA, Garber DA, et al. Flapless postextraction socket implant placement, part 2: the effects of bone grafting and provisional restoration on peri-implant soft tissue height and thickness—a retrospective study. Int J Periodontics Restorative Dent. 2015;35(6):803-809.
3. Tarnow DP, Chu SJ, Salama MA, et al. Flapless postextraction socket implant placement in the esthetic zone: part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change—a retrospective cohort study. Int J Periodontics Restorative Dent. 2014;34(3):323-331.
4. Saito H, Chu SJ, Reynolds MA, Tarnow DP. Provisional restorations used in immediate implant placement provide a platform to promote peri-implant soft tissue healing: a pilot study. Int J Periodontics Restorative Dent. 2016;36(1):47-52.
5. Waerhaug J. Tissue reactions around artificial crowns. J Periodontol. 1953;24(3):172-185.
6. Waerhaug J. Observations on replanted teeth plated with gold foil; reaction to pure gold; mode of epithelial attachment to gold; expulsion of foreign bodies from pockets. Oral Surg Oral Med Oral Pathol. 1956;9(7):780-791.
7. Waerhaug J. Tissue reaction around acrylic root tips. J Dent Res. 1957;36(1):27-38.
8. Elian N, Cho SC, Froum S, et al. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;19(2):99-104.
9. Chu SJ, Salama MA, Salama H, et al. The dual-zone therapeutic concept of managing immediate implant placement and provisional restoration in anterior extraction sockets. Compend Contin Educ Dent. 2012;33(7):524-532,534.
10. Chu SJ, Hochman MN, Tan-Chu JH, et al. A novel prosthetic device and method for guided tissue preservation of immediate postextraction socket implants. Int J Periodontics Restorative Dent. 2014;34 suppl 3:s9-s17.
11. Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121-135.
12. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008;19(7):635-641.
13. Abrahamsson I, Zitzmann NU, Berglundh T, et al. The mucosal attachment to titanium implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol. 2002;29(5):448-455.
14. Waerhaug J, Zander HA. Reaction of gingival tissues to self-curing acrylic restorations. J Am Dent Assoc. 1957;54(6):760-768.
15. Listgarten MA, Lai CH. Ultrastructure of the intact interface between an endosseous epoxy resin dental implant and the host tissues. J Biol Buccale. 1975;3(1):13-28.
16. Nevins M, Nevins ML, Camelo M, et al. Human histologic evidence of a connective tissue attachment to a dental implant. Int J Periodontics Restorative Dent. 2008;28(2):111-121.
17. Lowenberg BF, Pilliar RM, Aubin JE, et al. Migration, attachment, and orientation of human gingival fibroblasts to root slices, naked and porous-surfaced titanium alloy discs, and Zircalloy 2 discs in vitro. J Dent Res. 1987;66(5):1000-1005.
18. Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18(24):1573-1583.
19. Chehroudi B, Gould TR, Brunette DM. The role of connective tissue in inhibiting epithelial downgrowth on titanium-coated percutaneous implants. J Biomed Mater Res. 1992;26(4):493-515.
20. Kim H, Murakami H, Chehroudi B, et al. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int J Oral Maxillofac Implants. 2006;21(3):354-365.
21. Linkevicius T, Apse P. Biologic width around implants. An evidence-based review. Stomatologija. 2008;10(1):27-35.