Odontogenic Osteomyelitis or Bisphosphonate-Related Osteonecrosis of Mandible of Patient with Autoimmune Disease: Clinical Dilemma
Len Tolstunov, DDS, DMD; Darren P. Cox, DDS, MBA; and Bahram Javid, DMD
Abstract
The key to appropriate treatment of odontogenic osteomyelitis or bisphosphonate-related osteonecrosis of the mandible in patients with autoimmune diseases lies in making the correct diagnosis based on meticulous review of signs and symptoms. As this complex case involving a patient with multiple comorbidities illustrates, diagnosis can be difficult, because these conditions may overlap or be mistaken for other conditions. However, prompt treatment is essential to limit the progression, which can be devastating for these medically complex patients. It is, therefore, important to understand local and systemic conditions that can weaken the immune system and predispose patients to chronic bone infection, meticulously go through signs and symptoms, and have a complete medical history, including patient medications.
Chronic osteomyelitis (CO) is a rare infection of the medullary portion of the jawbone with purulent exudate. Of dental infectious origin, this condition often occurs in immunocompromised individuals. Chronically infected teeth can lead to a spread of infection “inward” to the vascular medullary bone, destroying it and forming an abscess. The infection then spreads both within the marrow and also through the cortical bone to the periosteum. The periosteum, with time, begins to form new bone (the involucrum) while the central necrotic bone is known to be called sequestrum. Both can be identified by x-ray after 6 to 8 weeks of the infectious process. Treatment of CO is surgical and includes excision of the soft-tissue sinuses, extraction of involved tooth or teeth, debridement of the intrabony abscess with removal of sequestra, and long-term antibiotic therapy (usually at least 6 to 8 weeks) based on the culture and sensitivity. An infectious disease consultation is often recommended.
Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is a relatively rare complication of treatment with nitrogen-containing bisphosphonates (NBPs), which are taken for a variety of conditions ranging from osteoporosis to metastatic cancer, resulting in avascular necrosis of the jawbone and bone exposure in the oral cavity. The exposed bone can become infected from pathogenic oral bacterial organisms. All bones of the skeleton are affected. Relative ease of bone exposure after extraction of a tooth contributes to the development of bone infection and necrosis. Due to a generalized involvement, this condition can also be called osteochemonecrosis. The bisphosphonate medications tend to selectively target and destroy osteoclasts, altering the bone metabolism and allowing a unilateral osteoblast-mediated bone build-up. This results in a formation of dense and poorly vascularized bone. This is why this disease can also be called “antiresorptive agent-induced ONJ (ARONJ).”1 Patients with cancer and metastatic disease receive intravenous (IV) bisphosphonates in significantly higher doses than patients with osteoporosis, who usually take oral medications. This leads to a disproportionately larger number of BRONJ cases among cancer and Paget’s disease patients taking IV bisphosphonates. Due to the involvement of the entire jaw, BRONJ cases tend to be treated more conservatively than cases of osteomyelitis or osteoradionecrosis, where surgical debridement is important.
Both CO and BRONJ can have similar signs and symptoms, are most commonly found in the posterior mandible, and result in the formation of necrotic bone in the jaws. Although there is no data on the interplay of multiple factors in these conditions, immunocompromised patients on long-term treatment with NBPs, who concurrently have an advanced dental disease, need to be evaluated for CO so an accurate diagnosis can be established and a targeted treatment plan implemented. In this report, the authors describe a challenging case involving a patient who developed jawbone necrosis. It emphasizes the importance of a proper differential diagnosis—including BRONJ and CO—while accurately determining the final diagnosis so that the proper therapy can be instituted.
The authors followed the guidelines of the Helsinki Declaration for this study. This article was exempt from the institutional review board approval.
Case Report
A 59-year-old woman was referred to the authors’ oral and maxillofacial surgery practice with a chief complaint of intermittent pain around the decayed roots of the third molar on her right side for the previous 2 months. The patient’s medical history was remarkable for rheumatoid arthritis (RA) and Crohn’s disease for which treatment included the following medications: azathioprine (Imuran®, GlaxoSmithKline, www.gsk.com): 100 mg once daily for about 5 years; mesalamine (Asacol®, Warner Chilcott Company, LLC, www.wcrx.com): 1600 mg three times daily for the previous 5 years; and prednisone: 12.5 mg daily for about 10 years. In addition, the patient had hypertension, right total hip replacement surgery without complications 3 years prior, and was allergic to penicillin.
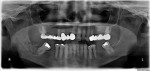
On examination, the patient had a decayed remaining lower right third molar root with associated mild swelling and erythema of the surrounding tissue. A panoramic radiograph showed a small portion of the third molar root mainly in the soft tissue above the alveolar bone level (Figure 1). The day after presentation, the patient underwent extraction of the root of the lower right third molar without complications. One hour prior to her surgery, the patient took 600 mg of clindamycin, and she continued on 300 mg of clindamycin three times daily for the next 5 days. The patient returned for follow-up examination 1 week postoperatively and demonstrated normal healing for this timeframe.
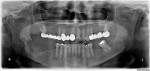
One month later, the patient called to report swelling and pain in the area of the extraction site. On examination, swelling was evident in the posterior mandible in the area of extraction, extending to the buccal vestibule and masseteric region, with limited mouth opening to about 15 mm, and purulent discharge behind the second molar. A subsequent panoramic radiograph revealed a mottled, irregular, mixed radiolucent/radiopaque appearance of the posterior mandible around the extracted site, which extended anteriorly to include the adjacent second molar (Figure 2). Given that this condition had existed for 6 to 8 weeks, the preliminary diagnosis of CO was made, and the patient consented to indicated treatment, which consisted of debridement of the wound and extraction of the second molar. The procedure was performed on the same day under local anesthesia; 600 mg of clindamycin was given just prior to the surgery. A buccal full-thickness flap in the second-third molar area was raised, and multiple small bony sequestra with granulations tissue were visualized and removed until fresh bleeding bone was seen; the involved second molar was also removed with copious irrigation after debridement. The patient was placed on clindamycin 300 mg four times daily and was followed weekly.
Four weeks later, the wound remained open with purulent discharge. A second, wider bone debridement procedure was performed, with removal of a few more necrotic bone fragments. Purulent discharge and necrotic bone were sent for culture and sensitivity testing, with later received results of a positive culture showing “mixed oral flora.” An infectious disease (ID) consultation was obtained, and the recommendation was to continue with another course of oral clindamycin and local treatment.
During that time, an important new piece of information was discovered by patient’s primary care physician: the patient had also been on alendronate (Fosamax®, Merck, www.merck.com) 70 mg once a week for the last 3 years due to concerns about potential osteoporosis in this post-menopausal woman on prednisone. “I forgot to mention this Sunday pill,” the patient revealed at the follow-up appointment. Joint consultation between the patient’s ID and primary care physicians resulted in a compromise approach to treating the jaw infection as well as continuing to treat her autoimmune conditions with the immunosuppressive therapy. The agreed upon approach was to discontinue the alendronate and to decrease the dosage of prednisone from 12.5 mg to 10 mg daily. The working diagnosis of the jaw condition at this point was BRONJ.
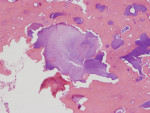
There was a temporary improvement in her jaw symptoms, but after 5 to 7 days, infection with purulent discharge returned. Follow-up imaging, including a panoramic radiograph (Figure 3), showed persistent mottled, mixed irregular radiolucent-radiopaque appearance, slightly more extensive than that seen on the previous panoramic image. The third debridement of the wound was performed 10 weeks after extraction of the root of the third molar, which was 6 weeks after the first debridement and 3.5 weeks after the second debridement and on day 43 of the oral clindamycin. A large amount of granulation tissue and multiple sequestra of devitalized bone were curetted out down to vital-appearing (bleeding) bone. A second culture and sensitivity test did not reveal the presence of microorganisms. The biopsy was positive for “necrotic sclerotic bone with acute osteomyelitis and colonies of filamentous bacterial organisms consistent with actinomycetes” (Figure 4). As actinomycotic organisms are present in the normal oral flora, the existence of actinomycetes is not an indication of “actinomycosis.” In addition, these features seen under the microscope do not clearly distinguish chronic osteomyelitis from BRONJ.
Because of concerns for mandibular osteomyelitis (and possibly actinomycosis), the ID physician discontinued oral clindamycin, and the patient was placed on a 6-week course of intravenous ceftriaxone (2 gm, once a day) through the peripherally inserted central catheter (PICC line). In 48 to 72 hours, the patient reported much improvement in symptoms. Granulation tissue began to fill the wound, negating the need for further debridement. After 6 weeks of ceftriaxone, complete closure of the wound was occurring, and a panoramic radiograph demonstrated significant remodeling of the bone without a mottled appearance or evidence of sequestration.
Although the patient subsequently developed diabetes mellitus (DM) type 2 and was placed on insulin, it did not complicate the wound healing (Figure 5). Upon discontinuation of ceftriaxone, the ID physician returned the patient back to a 3-week course of oral clindamycin (300 mg, three times a day). The last panoramic radiograph taken on this patient was 5 months after the initial presentation and 3 months after the last debridement. It demonstrated partial fill of the mandibular defect and bone remodeling without evidence of necrosis (Figure 6).
Discussion
In the described case, a patient with two autoimmune diseases (RA and Crohn’s disease)—who had been treated with alendronate sodium (Fosamax) and corticosteroid (prednisone)—developed necrotic bone (sequestra) in the mandible following extraction of a decayed third molar.
A few oral conditions can present as non-vital (necrotic) bone in the jaws, including: chronic (suppurative) osteomyelitis (CO), osteoradionecrosis, osteopetrosis, and bisphosphonate-related osteonecrosis of the jaws (BRONJ). Although any of these processes can develop in the immunocompetent individual, they can initiate much earlier and progress much more quickly in an immunocompromised patient or one with autoimmune disease on immunosuppressive medications, such as the patient in the described case report.
The patient in this case report had RA—a systemic chronic inflammatory autoimmune disease that usually occurs in middle-aged women and affects mainly joints and other tissues and organs (lungs, pericardium, sclera, etc.). It typically leads to symmetrical small-joint destruction (articular cartilage) and often ankylosis with functional compromise. Vasculitis compromising peripheral blood supply of tissues has an important role in pathophysiology of this condition. Treatment of RA includes anti-rheumatic drugs (Imuran or azathioprine, others), anti-inflammatory medications (glucocorticoids, NSAIDs), and analgesics (opioids, etc.).
The patient in the described case also had Crohn’s disease (regional enteritis, regional ileitis), an autoimmune gastrointestinal disease that primarily affects women and has common symptoms of severe abdominal pain, vomiting, diarrhea, weight loss, and other symptoms. Again, azathioprine, prednisone, and other medications are often used in Crohn’s disease.
The patient in the case report was on azathioprine (Imuran), an immunosuppressive drug, which was used in her case for both RA and Crohn’s disease. This medication is known to suppress the bone marrow, causing increased susceptibility of patients to infections. Another medication, mesalazine (5-aminosalicylic acid, Asacol) is an anti-inflammatory medication used to treat inflammatory conditions of the digestive tract, like Crohn’s disease. Finally, prednisone, as a systemic corticosteroid and immunosuppressive medication, was used to treat the patient’s chronic inflammatory conditions (RA and Crohn’s disease).
In the presented case, the patient developed necrotic bone with sequestration in the proximity of the decayed tooth. The tooth was eventually removed, but the infection spread deeper into the bone, resulting in the formation of necrotic bone and multiple sequestra. At this stage, the patient was diagnosed with chronic osteomyelitis.
The term osteomyelitis has been used to describe a wide variety of pathological conditions, including acute and chronic bacterial (suppurative), diffuse sclerosing, focal sclerosing (condensing osteitis), osteomyelitis with proliferative periostitis, and others. In this case, the patient was diagnosed with a classic pattern of chronic—often called bacterial, secondary, or suppurative to emphasize the infectious tooth-driven process—osteomyelitis, a rare “infection of the medullary portion of bone that includes the production of pus.”2 A patchy, mottled and “moth-eaten” appearance on the radiograph is typical for a chronic osteomyelitis (Figure 2). A prolonged course of oral and intravenous antibiotics is usually required to treat this condition.
Later into the case—when it became known that the patient had been on alendronate (nitrogen-containing bisphosphonate Fosamax) for the previous 3 years—the differential diagnosis included BRONJ. BRONJ or osteonecrosis of the jaw (ONJ), avascular jawbone necrosis, is related to use of NCBs by millions of patients for a range of conditions from osteoporosis to metastatic cancer. BRONJ is considered a risk for oral and especially intravenous bisphosphonate therapy.3-13 As much as chronic osteomyelitis, BRONJ on occasion can present with a chronic necrotic bone condition in the jawbone (usually not a sequester-type) that may look macroscopically (to a clinician) and microscopically (to a pathologist) similar, if not identical.
To differentiate BRONJ from other similar odontogenic conditions with delayed bone healing, the American Association of Oral and Maxillofacial Surgeons (AAOMS) has adapted the following working definition of BRONJ.14,15 Patients may be considered to have BRONJ if “all of the following three characteristics are present: 1) current or previous treatment with a bisphosphonate; 2) exposed bone in the maxillofacial region that has persisted for more than 8 weeks; and 3) no history of radiation therapy to the jaws.” According to the 2009 Position Paper of AAOMS,15 the two largest risk factors for BRONJ were identified as bisphosphonate medication (usually, intravenous) and dentoalveolar surgery. Duration of bisphosphonate therapy plays an important role and the mandible appears to be a favorite location for this condition.14,15
The above description by the AAOMS Taskforce can be applicable for the diagnosis of BRONJ in the presented case. In this patient: 1) the mandibular bone necrosis developed after about 3 years on alendronate, which the patient was still taking at the time of presentation; 2) when the tooth was removed—dentoalveolar procedures are a major risk factor—she developed necrotic bone that was exposed to the oral cavity; 3) the infectious process persisted for more than 8 weeks; and 4) there was no history of radiation therapy.
The same patient’s clinical and radiographic presentation would also be consistent with chronic osteomyelitis in meeting these criteria: There was 1) an obvious infection of the medullary bone of the posterior mandible with purulent discharge of a long duration associated with a decayed (infected) tooth; 2) the presence of multiple sequestra; 3) the need for multiple bone debridement procedures; and 4) a long course of oral and intravenous antibiotic treatment that was necessary to control infection.
The clinical dilemma in cases such as these—which is clear from the title of this article—is to formulate the proper diagnosis. Was it chronic osteomyelitis or BRONJ, or was it both pathological processes? Did this patient have an infection of the medullary compartment of mandible with development of non-vital bone (sequestration) that was initiated from and related to the decayed third molar and worsened by immunosuppressive medications (for treatment of RA and Crohn’s disease); or did the patient have a chronic bisphosphonate-mediated osteoclast inhibition of bone resorption and related decrease of bone turnover1 exacerbated by taking corticosteroid treatment (prednisone)—given the known risk associated with nitrogen-containing bisphosphonate therapy and characteristic of BRONJ?
Making the Diagnosis
The authors believe that in this particular case, the proper diagnosis tends to point towards osteomyelitis, with BRONJ playing the secondary role. There are several subtle clinical indicators that were important in the learning process and that led to this conclusion.
First, careful inspection of the original panoramic radiograph at the patient’s presentation (Figure 1) revealed the existence of the periapical mottled radiolucent/radiopaque lesion, indicating the initiation of bone infection even prior to extraction of the decayed third molar. This is common for jaw osteomyelitis of odontogenic origin (from the infected tooth of a long duration) and not common for BRONJ, where the dentoalveolar surgery is usually the initiating factor (a major risk factor, according to the 2009 AAOMS Position Paper).15
Next, it is known that as the treatment of chronic osteomyelitis progresses, the surrounding vital healthy bone develops a protective involucrum and undergoes the remodeling process with a slow return of the typical trabecular pattern of bone. This was seen in the described case (Figure 4). Presence of a vital surrounding bone was also confirmed clinically every time the debridement procedure was performed. BRONJ treatment often does not show signs of remodeling and separation due to presence of permanently affected avascular bone in the entire jaw. In BRONJ cases, the entire skeleton is affected by the drug-related condition (often called chemonecrosis). The morning fasting serum C-terminal cross-linking telopeptide (CTX), an index of bone turnover that can help to identify the degree of BRONJ, was not tested in the presented case.
A third indicator is that jaw osteomyelitis usually resolves with an aggressive surgical debridement and oral/intravenous antibiotic therapy16; this occurred in the presented case. Treatment of BRONJ is often more complicated and currently not clear. It may slowly improve with a conservative superficial debridement of just exposed portions of necrotic bone. That was not the approach that was used in the presented case.
Fourth, targeted antibiotic therapy is extremely helpful in treatment of CO. In the presented case, although oral clindamycin was not effective initially, the patient dramatically improved as soon as targeted intravenous ceftriaxone therapy was initiated. The role of antibiotic therapy in BRONJ cases is less clear and less successful. Due to the avascular state of the surrounding bone and a decrease in bone turnover, an antibiotic may not always get to the affected jaw area and a topical (rinses) use of chlorhexidine is often more practical.
Finally, the association between an immunocompromised systemic condition and development of osteomyelitis is strong. Osteomyelitis of the jaw is often accompanied by regional or systemic compromise of the immune response.16 In this patient with RA and Crohn’s disease, immunosuppressive medication (Imuran) and corticosteroid prednisone played an important role in the weakening of the patient’s immune response, causing increased susceptibility to infections and development of CO. Vasculitis (small vessel disease), compromising peripheral blood supply of tissues, also plays an important role in pathophysiology of both RA and Crohn’s disease. The link between the immunocompromised state of a host and BRONJ is not clear at this stage.17
Finally, a statistical occurrence of BRONJ in patients on long-term oral bisphosphonate is below 0.1%.15,18 The patient in the report had only about 3 years of exposure to the bisphosphonate therapy. Although it is rare to see CO of the jaws in developed countries, it has been described in patients with immune comorbidities.16,19
It also important to mention that the presence of both conditions in one patient is possible. Saia et al20 reported BRONJ cases as a rare outcome in high-risk nitrogen-containing bisphosphonate users who underwent surgical extraction where baseline osteomyelitis was a strong risk factor for BRONJ development.
Summary and Conclusion
In summary, it appears that in the presented case the patient’s jawbone was significantly altered by NBPs and the BRONJ process. This patient developed an unfortunate combination of both a systemic (three immune comorbidities—RA, Crohn’s, and DM) and local (BRONJ) predisposition for the development of a deep necrotic bone infection, a chronic bacterial osteomyelitis initiated by the decayed third molar.
In conclusion, the authors believe that it is extremely important for oral and maxillofacial surgeons to understand local and systemic conditions that can weaken the immune system and predispose dental patients to chronic bone infection. It was fascinating for the authors to carefully sort out the history of the present illness(es), as well as to meticulously go through signs and symptoms in the complex case with multiple comorbidities to come up with a correct diagnosis that lead to a proper and targeted treatment plan that culminated in the patient’s complete recovery.
Acknowledgment
The authors thank Stephen E. Follansbee, MD, an infectious disease specialist, for review of the article and thoughtful recommendations.
References
1. Hellstein JW, Adler RA, Edwards B, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142(11):1243-1251.
2. Marx RE, Stern D, eds. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Hanover Park, IL: Quintessence Publishing; 2003:54-57.
3. Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy wpamidronate for the treatment of hypercalcemia of malignancy: Comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993;95(3):297-304.
4. Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled, clinical trials. J Clin Oncol. 2001;19(2):558-567.
5. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Eng J Med. 1996;335(24):1785-1791.
6. Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16(6):2038-2044.
7. Hillner BE, Ingle JN, Chelbowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042-4057.
8. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic [Letter]. J Oral Maxillofac Surg. 2003;61(9):1115-1117.
9. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff S. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527-534.
10. Estilo CL, Van Poznak CH, Wiliams T. Osteonecrosis of the maxilla and mandible in patients treated with bisphosphonate therapy. Oncologist. 2008;13(8):911-920.
11. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis\osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg. 2005;63(11):1567-1575.
12. Dodson TB. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):44-52.
13. Marx RE. Oral and Intravenous Bisphosphonate-Induced Osteonecrosis of the Jaws. Hanover, Park, IL: Quintessence Publishing; 2007.
14. Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369-376.
15. Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2-12.
16. Hudson JW. Osteomyelitis of the jaws: a 50-year perspective. J Oral Maxillofac Surg. 1993;51(12):1294-1301.
17. Conte-Neto N, Bastos AS, Spolidorio LC, et al. Oral bisphosphonate-related osteonecrosis of the jaws in rheumatoid arthritis patients: a critical discussion and two case reports. Head Face Med. 2011;27(7):7.
18. Lo J, O’Ryan F, Gordon N, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243-253.
19. Bernier S, Clermont S, Maranda G, Turcotte JY. Osteomyelitis of the jaws. J Can Dent Assoc. 1995;61(5):441-442, 445-448.
20. Saia G, Blandamura S, Bettini, G, et al. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68(4):797-804.
About the Authors
Len Tolstunov, DDS, DMD
Oral and Maxillofacial Surgery
Private Practice
San Francisco, California
Assistant Professor
Department of Oral and Maxillofacial Surgery
University of the Pacific
Arthur A. Dugoni School of Dentistry
San Francisco, California
Assistant Professor
UCSF
School of Dentistry
San Francisco, California
Darren P. Cox, DDS, MBA
Associate Professor
Department of Oral and Maxillofacial Pathology
University of the Pacific
Arthur A. Dugoni School of Dentistry
San Francisco, California
Bahram Javid, DMD
Associate Professor
Department of Oral and Maxillofacial Surgery
University of the Pacific
Arthur A. Dugoni School of Dentistry
San Francisco, California