Two-Year Evaluation Indicates Zirconia Bridges Acceptable Alternative to PFMs
Ronald D. Perry, DMD, MS; Gerard Kugel, DMD, MS, PhD; Shradha Sharma, DMD; Susana Ferreira, DMD; and Britta Magnuson, DMD
Abstract
OBJECTIVE: The aim of this in-vivo study was to evaluate the 2-year clinical performance of zirconia computer-aided design/computer-aided manufacturing (CAD/CAM)-generated bridges.
METHODS: A total of 16 three- or four-unit Lava zirconia bridges were done on 15 subjects. The bridges were cemented using RelyX™ Unicem Self-Adhesive Universal Resin Cement. Evaluation was done at 6-month, 1-year, and 2-year recall visits. Evaluation criteria were color stability and matching, marginal integrity, marginal discoloration, incidence of caries, changes in restoration–tooth interface, changes in surface texture, postoperative sensitivity, maintenance of periodontal health, changes in proximal and opposing teeth, and maintenance of anatomic form. In each of these parameters, the bridges were rated in one of three possible categories: “A” (alpha)–ideal; “B” (bravo)–acceptable; and “C” (charlie)–unacceptable.
RESULTS: After 2 years, 100% of the bridges were rated “A” for color stability and matching, marginal discoloration, incidence of caries, changes in restoration–tooth interface, changes in surface texture, postoperative sensitivity, and change in proximal or opposing teeth. In the parameter of marginal integrity, 6.25% of the bridges were rated “B;” the remaining 93.75% were rated “A.” Maintenance of periodontal health was rated “B” for 6.25% of the bridges and “A” for 93.75%. At 2 years, 12.5% of the bridges rated “C” in maintenance of anatomic form and 87.5% rated “A.”
CONCLUSION: The overall clinical outcome was that the CAD/CAM-generated zirconia bridges were clinically acceptable.
The search for a strong yet esthetic material with which to fabricate fixed partial dentures (FPDs) has been ongoing for many years. For most of this time the standard has been porcelain-fused-to-metal (PFM). PFMs have been used in dentistry for about the past 50 years, ever since developments enabled the fusion of porcelain to gold alloys.1,2 In addition to PFMs, ceramic systems have been studied for use as FPDs. A number of ceramic systems have been developed for use in dentistry, some of which work in the anterior esthetic area but have proven too weak for posterior use. For example, lithium disilicate is limited to use in bridges that replace a tooth anterior to the second premolar.3 Other ceramics have proven strong enough for posterior applications, but are not desirable from an esthetic standpoint. For instance, In-Ceram Zirconia® (Ivoclar Vivadent, Inc., www.ivoclarvivadent.us), a glass-infiltrated alumina with 35% partially stabilized zirconia, is not recommended for fabricating anterior all-ceramic FPDs.4 This is due to its lack of translucency, which actually is similar to that of PFM restorations.5 While these and other ceramic systems have dental uses, zirconia is a ceramic that combines strength, esthetics, and ease of manufacturing into one material allowing for posterior, esthetic FPDs.
Zirconia (zirconium oxide, ZrO2) is the white crystalline powder oxide of the naturally occurring metal zirconium.1 Research into the medical uses of zirconia dates back to the late 1960s.6 However, its use in dentistry did not start until the 1990s in the form of endodontic posts7 and implant abutments.8
The high flexural strength of zirconia—especially in comparison to other dental ceramics—has been documented.9 The strength of zirconia allows for a reduction in connector size as compared to other dental ceramic systems.10 Other dental ceramics often fail in the connector area, which leads to a need for an increase in connector size.11 The smaller connector size that zirconia allows for is desirable for both esthetic and periodontal reasons.
The biocompatibility of zirconia has been widely studied and the material has been found to be tissue-compatible. Research has also shown that there were no local or systemic adverse reactions or cytotoxic effects noted.6,12,13 Additionally, zirconia crowns and bridges are metal-free, which is an attractive feature in light of an increased demand among patients for metal-free restorations. One reason for this is concern over metal allergies. While allergies to dental alloys have been studied and are not common,14 there are patients for whom allergies are a concern. Because sensitivities and allergies do not appear to be a problem encountered with its use in dentistry, zirconia offers a biocompatible alternative.12
PFMs are considered to be the standard by which to measure new systems for use as crowns and bridges. In comparison to PFMs, the strength of zirconia allows for a reduction in the thickness of the core material, which is beneficial from an esthetic viewpoint.15 Also, zirconia’s marginal fit has been studied and found to compare well with PFM crowns and bridges.16 As previously mentioned, the strength of zirconia allows for a smaller connector size, which approaches that of PFMs.10
It is clear that many characteristics of zirconia lead to its being considered an exceptional dental material. Research has been conducted to study how well zirconia performs as a framework for posterior bridges.17-20 These studies have shown that zirconia bridges are a clinically acceptable alternative to PFMs. The objective of this study was to assess the clinical efficacy and longevity of zirconia bridges.
Methods and Materials
Patient Selection
Fifteen subjects—five men and 10 women—21 years of age or older were selected for the study. Each was required to exhibit a low to moderate caries rate, Class I periodontal condition, and uncompromised dentition that showed no abnormal wear patterns. Excluded from the study were those with severe medical complications (organ transplants, cancer, immunocompromised, or undergoing long-term antibiotic or steroid therapy), history of compromising systemic disease, current drug use, pregnant, involved in the evaluation of other dental materials, history of adverse reaction to clinical materials of the types used in the evaluation, or severe salivary gland dysfunction.
A baseline clinical examination was completed. Suspected cases of previous carious exposure were not included unless successful endodontic treatment had been completed. Soft tissues were examined to determine if any pathology was present. A baseline periapical radiograph was taken of each tooth; only vital teeth that appeared normal on the radiograph or teeth treated successfully with endodontic therapy were selected for the study.
Clinical Procedures
An informed consent form was signed by each patient at enrollment. After the subjects’ medical and dental histories were reviewed, preoperative clinical photographs were taken. Prior to tooth preparation, a shade was chosen using the VITA Classical Shade Guide (Vident, A VITA Company, www.vident.com).
Bridge preparation followed these guidelines: Subjects were administered local anesthetics using 2% lidocaine with epinephrine 1:100,000 or 3% carbocaine in order to render profound anesthesia. All bridge margins were placed at the gingival crest, except where the requirements of retention or esthetics dictated a subgingival preparation. In those cases where a subgingival finish line was placed, the biologic depth of the sulcus was not violated in order to ensure maintenance of the periodontal tissue.
All bridges were prepared starting with No. 4 diamond burs for depth cuts, and the remaining tooth structure was reduced with a No. 773 diamond to 12-degree convergence of the preparation. Occlusal and facial reduction was 1.5 mm to 2 mm, while interproximal and lingual reduction was approximately 1.5 mm. The facial finish line for esthetics was a butt joint with a 1.5 mm to 2 mm shoulder prepared.
After a suitable preparation was made, gingival retraction was done with either a nonepinephrine- or epinephrine-containing cord to initiate the impression procedure. Impressions of the upper and lower arches were made using a polyether impression material and a full-arch impression tray. Occlusal registration was recorded using bite registration material.
A clinical photograph of the preparation was taken. The preparations were temporized using bis-acryl. The impressions and bite registration were sent to the laboratory for fabrication of the Lava™ bridge (3M ESPE, www.3Mespe.com).
A framework try-in was done to check for the fit and marginal integrity using a 17/23 dental explorer. After the Lava Ceram Generation 1 (3M ESPE) was applied at the lab, the bridge was returned for final cementation.
Insertion
The bridges were checked for fit and marginal integrity with a 17/23 dental explorer and bitewing radiographs. After the fit and margins were judged acceptable, the bridges were evaluated and adjusted for occlusion, interproximal contact, and shade match. Appropriate changes were made, if needed, and the bridges were cemented using RelyX™ Unicem Self-Adhesive Universal Resin Cement (3M ESPE). Following cementation, polyvinyl siloxane impressions of the bridges were taken, as was a photograph of the cemented bridge.
Follow-ups were at 6 months, 1 year, and 2 years. The following parameters were rated: color stability and matching, marginal integrity, marginal discoloration, incidence of caries, changes in restoration–tooth interface, changes in surface texture, postoperative sensitivity, maintenance of periodontal health, changes in proximal and opposing teeth, and maintenance of anatomic form. The parameters were each rated in one of three possible categories: “A” (alpha)–ideal; “B” (bravo)–acceptable; and “C” (charlie)–unacceptable (Table 1). In addition, a photograph was taken.
Results
The results of this study support the evidence that zirconia is an appropriate material for anterior and posterior bridges. Most parameters were rated “A” at all visits. There were instances of less than ideal maintenance of periodontal health. While subjects were excluded from the study if they had moderate/severe periodontitis, some of the subjects did have poor oral hygiene habits. Two of the bridges rated “B–acceptable” at both the 6-month and 1-year visits subsequently improved to “A” by the 2-year visit. The other bridges that scored “B” only received that score at one of the three follow-up visits. This suggests that instead of a chronic condition caused by the bridge itself, a lack of oral hygiene caused the “B” ratings and after the subjects improved their oral hygiene, there was an improvement in periodontal health.
Sensitivity was found in one bridge at both the 6-month and 1-year visits. At both of these visits, desensitizer was applied and a follow-up phone call was performed to ensure the subject was no longer experiencing sensitivity. The desensitizer was effective each time it was applied, and by the 2-year visit, the sensitivity had completely subsided.
The marginal integrity of all the bridges was “A” at 6-month and 1-year recall visits. At the 2-year visit, one bridge scored “B” for marginal integrity. It is possible that a small amount of cement had leached out, leaving a slight area in which the explorer could catch.
Maintenance of anatomic form was “A” for all the bridges at the 6-month recall visit. At the 1-year visit, one bridge was rated “C” for chipping and the rest were rated “A.” By the 2-year visit, one other bridge exhibited chipping and was rated “C,” thus bringing the total to two bridges rated “C” in anatomic form and 14 rated “A.” In both of these instances the bridges were polished, because the chips were minor and the subjects did not wish to replace the bridges.
Discussion
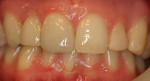
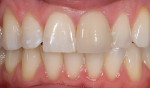
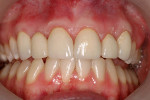
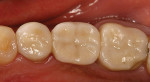
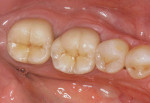
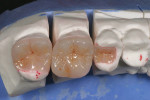
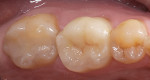
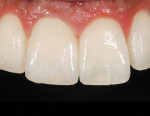
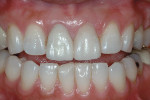
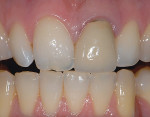
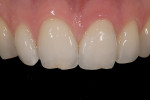
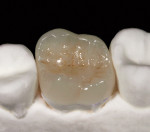
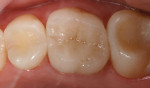
The current literature and recent reports seem to support zirconia as a clinically acceptable material for posterior bridges.17-20 However, it should be noted that many of the studies are still short-term (up to 5 years), as the use of zirconia for this application is relatively new, and long-term longitudinal studies are still needed. This study joins the previous studies in being a short-term trial to observe the effectiveness of zirconia as a framework for posterior FPDs as well as anterior bridges (Figure 1, Figure 2 and Figure 3).
Framework fracture of zirconia does not appear to be a problem11 as it is in other ceramic systems and it was not found in this study. This is likely due to the mechanical properties of zirconia, including strength9 and a phenomenon known as “transformation toughening.”21 Zirconia occurs in three forms—monoclinic, cubic, and tetragonal.22 Yttrium oxide can then be added to the tetragonal form in order to stabilize it, therefore making yttrium tetragonal zirconia polycrystals (Y-TZP).19 Transformation toughening occurs when the material is under external stresses and the material transforms from the tetragonal phase to the monoclinic phase.21 This transformation leads to a 4% increase in volume, which in effect clamps/seals the crack and does not allow it to propagate.21
The strength of zirconia following long-term exposure to a moist environment has been a topic of research. The conclusions of these studies have differed, ranging from finding a dramatic loss of strength in zirconia under moist conditions23 to a study finding that discs of Lava zirconia do not exhibit a decrease in strength after storage in water.24 While studies have found a decrease in zirconia’s strength after aging in water,23,25 it has also been concluded that, due to zirconia’s high mechanical properties, it is still the most suitable ceramic for posterior bridges.25
Zirconia must be used in the appropriate clinical situations, and, in some instances, PFMs are still the best option for restoring a tooth. One contraindication for the use of zirconia is heavy bruxism.3 Also, good periodontal health and lack of mobility of the abutment tooth are needed to ensure a good outcome with a zirconia bridge.3
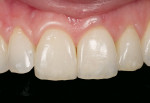
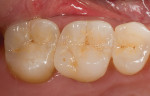
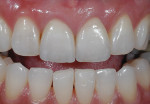
An issue encountered in this study was chipping of the veneer in two bridges (Figure 4). Chipping of porcelain veneering has been reported in other studies.20,26 Indeed, chipping seems to be the primary problem encountered with zirconia bridges. This is in contrast to PFMs, which have a lower rate of problems with the porcelain veneering. Studies of gold alloy PFMs have found porcelain problems in 4% of cases after 10 years27 and in 2% of cases after 5 years.28 Problems with porcelain in zirconia systems are reported at a higher rate than gold alloy PFMs. In this study 12.5% of cases had chipping problems after 2 years; in another study, 15% had chipping problems in 2 years.29
Studies have found the porcelain chipping of zirconia bridges is due to tensile stresses.15,30 Studies have also shown that chipping occurs due to the fact that mechanical properties of the porcelain, such as fracture toughness and flexural strength, are not as great as those of zirconia.26 Another possible cause relates to a difference in thermal expansion coefficients between the zirconia and the veneering porcelain,31 although some studies suggest that this is not a likely cause.15 Regardless, newer porcelains were developed to be more compatible with zirconia’s thermal expansion coefficient, and it is possible that the earlier porcelains were in need of further development.
It is important to note that PFM crowns and bridges have been in existence for approximately 50 years and there has been time to develop reliable techniques for their production. Zirconia is still a newer product in the dental field, and techniques are still being developed to ensure the optimal outcome. Zirconia has great potential, and with time to develop the necessary techniques, it may become as reliable or as close to reliable as PFMs.
This study was completed in 2006, and since then there have been no complaints regarding any of the bridges placed. The next step will be a 6-year follow-up of the bridges. At that visit, the bridges will be rated using the same parameters and the same criteria. It is not expected that there will be any problems encountered with the bridges.
Conclusion
The zirconia all-ceramic restorations have demonstrated improved flexural, high-strength biocompatibility and esthetic characteristics. Zirconia bridges demonstrated a very high long-term mechanical reliability in 2-year follow-ups when proper selection, preparation, and fabrication procedures were followed. After 2 years, only two out of 16 bridges were unacceptable due to failure in the evaluation criteria, namely, maintenance of anatomic form. The results from this study indicate that zirconia bridges are an acceptable alternative to PFMs. While zirconia bridges do not replace PFMs at this time, they do expand a dentist’s choices when determining the best treatment option for a patient. The overall clinical outcome of the CAD/CAM-generated Y-TZP bridges was clinically acceptable.
Disclosure
This study was supported in part by 3M.
References
1. Christensen GJ. Porcelain-fused-to-metal versus zirconia-based ceramic restorations, 2009. J Am Dent Assoc. 2009;140(8):1036-1039.
2. Kelly JR, Nishimura I, Campbell SD. Ceramics in dentistry: historical roots and current perspectives. J Prosthet Dent. 1996;75(1):18-32.
3. Raigrodski AJ. Contemporary materials and technologies for all-ceramic fixed partial dentures: a review of the literature. J Prosthet Dent. 2004;92(6):557-562.
4. McLaren EA. All-ceramic alternatives to conventional metal-ceramic restorations. Compend Contin Educ Dent. 1998;19(3):307-312.
5. Heffernan MJ, Aquilino SA, Diaz-Arnold AM, et al. Relative translucency of six all-ceramic systems. Part I: core materials. J Prosthet Dent. 2002;88(1):4-9.
6. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20(1):1-25.
7. Meyenberg KH, Lüthy H, Schärer P. Zirconia posts: a new all-ceramic concept for nonvital abutment teeth. J Esthet Dent. 1995;7(2):73-80.
8. Wohlwend A, Studer S, Schaerer P. The zirconium oxide abutment: an all-ceramic abutment for the esthetic improvement of implant superstructures. In: Sorensen JA, ed. Quintessence of Dental Technology. Vol. 20. Carol Stream, IL: Quintessence Publishing; 1997:63-74.
9. Tinschert J, Zwez D, Marx R, Anusavice KJ. Structural reliability of alumina-, feldspar-, leucite-, mica- and zirconia-based ceramics. J Dent. 2000;28(7):529-535.
10. Giordano R. Materials for chairside CAD/CAM-produced restorations. J Am Dent Assoc. 2006;137 suppl:14S-21S.
11. Sailer I, Pjetursson BE, Zwahlen M, Hämmerle CH. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin Oral Implants Res. 2007;18 suppl 3:86-96.
12. Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68(2):322-326.
13. Covacci V, Bruzzese N, Maccauro G, et al. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999;20(4):371-376.
14. Geurtsen W. Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med. 2002;13(1):71-84.
15. Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24(3):299-307.
16. Reich S, Wichmann M, Nkenke E, Proeschel P. Clinical fit of all-ceramic three-unit fixed partial dentures, generated with three different CAD/CAM systems. Eur J Oral Sci. 2005;113(2):174-179.
17. Beuer F, Edelhoff D, Gernet W, Sorensen JA. Three-year clinical prospective evaluation of zirconia-based posterior fixed dental prostheses (FDPs). Clin Oral Investig. 2009;13(4):445-451.
18. Schmitter M, Mussotter K, Rammelsberg P, et al. Clinical performance of extended zirconia frameworks for fixed dental prostheses: two-year results. J Oral Rehabil. 2009;36(8):610-615.
19. Crisp RJ, Cowan AJ, Lamb J, et al. A clinical evaluation of all-ceramic bridges placed in UK general dental practices: first-year results. Br Dent J. 2008;205(9):477-482.
20. Sailer I, Fehér A, Filser F, et al. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20(4):383-388.
21. Guazzato M, Albakry M, Ringer SP, Swain MV. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent Mater. 2004;20(5):449-456.
22. Garvie RC, Hannink RH, Pascoe RT. Ceramic Steel? Nature. 1975;258:703-704.
23. Tinschert J, Natt G, Mohrbotter N, et al. Lifetime of alumina- and zirconia ceramics used for crown and bridge restorations. J Biomed Mater Res B Appl Biomater. 2007;80(2):317-321.
24. Curtis AR, Wright AJ, Fleming GJ. The influence of simulated masticatory loading regimes on the bi-axial flexure strength and reliability of a Y-TZP dental ceramic. J Dent. 2006;34(5):317-325.
25. Studart AR, Filser F, Kocher P, Gauckler LJ. In vitro lifetime of dental ceramics under cyclic loading in water. Biomaterials. 2007;28(17):2695-2705.
26. Raigrodski AJ, Chiche GJ, Potiket N, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006;96(4):237-244.
27. Anderson RJ, Janes GR, Sabella LR, Morris HF. Comparison of the performance on prosthodontic criteria of several alternative alloys used for fixed crown and partial denture restorations: Department of Veterans Affairs Cooperative Studies project 147. J Prosthet Dent. 1993;69(1):1-8.
28. Walter M, Reppel PD, Böning K, Freesmeyer WB. Six-year follow-up of titanium and high-gold porcelain-fused-to-metal fixed partial dentures. J Oral Rehabil. 1999;26(2):91-96.
29. Vult von Steyern P, Carlson P, Nilner K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. J Oral Rehabil. 2005;32(3):180-187.
30. Swain MV. Unstable cracking (chipping) of veneering porcelain on all-ceramic dental crowns and fixed partial dentures. Acta Biomater. 2009;5(5):1668-1677.
31. Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007;35(11):819-826.
About the Authors
Ronald D. Perry, DMD, MS
Director and Clinical Professor
Gavel Center for Restorative Dental Research
Tufts University School of Dental Medicine
Boston, Massachusetts
Associate Dean for Research,
Professor of Prosthodontics and Operative Dentistry
Tufts University School of Dental Medicine
Boston, Massachusetts
Shradha Sharma, DMD
Private Practice
Billerica, Massachusetts
Susana Ferreira, DMD
Adjunct Assistant Professo
Tufts University School of Dental Medicine
Boston, Massachusetts
Britta Magnuson,
DMD Instructor,
Tufts University School of Dental Medicine
Boston, Massachusetts