Long-Term Use of 6.5% Hydrogen Peroxide Bleaching Strips on Tetracycline Stain: A Clinical Study
Gerard Kugel, DMD, MS, PhD; Robert W. Gerlach, DDS, MPH; Ayman Aboushala, DMD, MS; Susana Ferreira, DMD; and Britta Magnuson, DMD
Abstract
This study evaluated the clinical response following extended daily use of a trayless 6.5% hydrogen peroxide (H2O2) tooth bleaching gel in patients with tetracycline stains. MATERIALS AND METHODS: In this clinical trial, participants (N = 30) were assigned to a strip product following screening for tetracycline staining. Subjects used the product for 6 months, with clinical safety and efficacy measurements assessed at the end of each month. The strips were used for 30 minutes twice daily (maxillary arch only). Treatment efficacy was determined by the tooth color change from baseline using an expanded VITA shade guide. Additional shades were added on either side, representing colors whiter than B1 and darker than C4. RESULTS: The strips averaged shade reduction in Months 1, 2, and 3 visits, respectively. Of all the subjects, 65% reached B1 color by Month 6. Treatment was generally well tolerated. Mild and transient tooth sensitivity (46.7%) and oral discomfort (43.3%) were the most common adverse events associated with daily bleaching, yet neither affected study participation. CONCLUSIONS: The 6.5% H2O2 bleaching strip provided significant tooth whitening in subjects with tetracycline stains. CLINICAL SIGNIFICANCE: When used daily for 6 months, a 6.5% H2O2 bleaching strip can be effective in whitening tetracycline stains. The professional strip was well tolerated throughout the 6-month period. This extended use provides additional evidence of clinical safety associated with the use of this product for vital bleaching.
Aging, chromatogenic food and drinks, excessive fluoride, medication (eg, tetracycline), and other agents can cause intrinsic stains. Tetracycline stains, though, are considered to be the most difficult to manage clinically. The devastating effect on tooth formation with as little as 1 g of tetracycline was recognized in the late 1950s1 and confirmed by Swachman et al in a study of patients with cystic fibrosis.2 In 1970, Cohen and Parkins published a method for in-office bleaching of the discolored dentin of young adults with cystic fibrosis who had received tetracycline treatment.3
Different variations of tetracycline and derivates produce various tooth colors. When the teeth are exposed to sunlight, they darken, with a distinct gray/blue tinge. It is suggested that the reason the front incisor teeth darken while the molars remain yellow longer is the different exposure to light.3 The discoloration of the permanent dentition depends on the amount and duration of tetracycline use.4 The portion of the tooth, color, and severity of the stains can be determined by the stage of tooth development when the drug was administered. Tetracycline is incorporated into dentin during tooth calcification intra- and postpartum, probably through chelation with calcium-forming tetracycline orthophosphate.5 It can be deposited in fetal tooth buds when administered in the third trimester or in a child in tooth development (ages 3 to 4 months and 7 to 8 years).6,7 A few reports state that long-term use of minocycline can stain adult teeth.8 The mechanism of minocycline permanently staining teeth is not well understood; some studies show that minocycline might bind to iron or plasma proteins.9
Colors may vary in intensity of gray, blue, brown, and yellow. The different banding can also be attributed to the variations of the tetracycline derivates. Tetracycline-stained teeth may respond to bleaching treatments but at a different rate than staining from other sources.10,11 Treatment is usually achieved by using at-home vital bleaching kits, which were introduced in 1989.12 The most common regimen in the literature involves overnight application of the bleaching tray for several months.10 Even with this extended treatment time, clinical outcomes may be mixed.
Recently, a strip-based system was developed to deliver 6.5% hydrogen peroxide (H2O2) topically without a custom tray.13,14 This system was used 30 minutes twice daily. The research was conducted to extend strip treatment for a longer duration than in previous studies. This was performed in order to evaluate clinical safety and effectiveness of vital bleaching in patients during a longer period.
Materials and Methods
The clinical research evaluated clinical safety and effectiveness of an H2O2-based tooth whitening system under conditions of extended daily use in patients with tetracycline-stained teeth. Participants used 6.5% H2O2 whitening strips (Crest® Professional Whitestrips, Procter & Gamble, www.pg.com) and were evaluated by the researchers.
Bleaching was performed daily for 6 months. Cases with tetracycline staining were targeted because they are widely recognized to require several months of treatment.15 Eligibility was limited to healthy adults who had at least 16 natural teeth, including all four maxillary central and lateral incisors, and at least three gradable maxillary incisors having clinical evidence of tetracycline-related staining. Individuals who had previously bleached their teeth and those with untreated periodontal disease, dental caries, or dentinal hypersensitivity were excluded. Subjects who were pregnant were not excluded as the manufacturer does not list this as a contraindication.
The protocol, informed consent, and advertising were reviewed and approved by Tufts University Institutional Review Board. Written and verbal informed consent was obtained from each subject prior to initiating the study. Because the tray system had been used for an extended period in previous studies and shown to be safe, this research was specifically designed to thoroughly evaluate the clinical safety and effectiveness of the strip system under extended usage conditions.16
Each kit contained one carton of 56 upper strips in pouches. In addition to the test products, all subjects were supplied with an anticavity dentifrice (Crest Cavity Protection, Procter & Gamble) and two soft-bristled toothbrushes (Crest Complete, Procter & Gamble). All study-related products and materials were dispensed every 4 weeks in nonidentifiable kits with unique subject identification numbers.
Only the maxillary arch was treated. Strip use was 30 minutes twice daily. Because only the maxillary arch was treated, all participants were offered a marketed, peroxide-based tooth whitening system for use on the mandibular arch after study completion.
Clinical response was evaluated at baseline and every 4 weeks during treatment. The level of tetracycline stain on the maxillary anterior teeth was assessed using a modified standard index (Jordan and Boksman Tetracycline Stain Classification) that recognized the possibility of successfully bleaching teeth with relatively severe tetracycline stain17,11 (Table 1).
Efficacy was assessed using a standard 16-tab value-oriented tooth shade guide (VITA Lumin®, Vita Zahnfabrik, www.vita-zahnfabrik.com). Two trained and calibrated examiners performed shade examinations under color-balanced lighting conditions. Both evaluators were calibrated at 85% rater reliability in determining shades before the onset of this project. Calibration was conducted pre- and post-study to ensure inter- and intra-rater reliability. Examiners rated the shade independently and the score was then averaged. Safety was assessed at baseline and each subsequent visit. Subjects were interviewed each time to ascertain the occurrence of any tooth hypersensitivity and/or oral irritation during treatment.
All adverse events were recorded irrespective of cause. For individuals with positive findings, severity and duration were assessed, along with any interventions (eg, rescue medications, treatment alteration) in accordance with pharmaceutical research standards.
Where banding was present, measurements were based on the predominant shade of the tooth outside the banded zone. Individual shade scores were determined by ranking the 16 shade tabs, arranged from light to dark, according to the rank order suggested by the manufacturer. This value-ordered ranking assigned “B1” as the first (lowest) step and “C4” as the last (highest) step of the 16 possible outcomes. To account for exceedingly light or dark tooth colors in the present study, researchers added two categories to represent colors lighter or darker: shade “B1–” (whiter than “B1”) with numeric code “0,” and shade “C4+” (darker than “C4”) with numeric code “17.”
Effectiveness was determined by calculating the change in shade scores from baseline at each visit. Using the expanded 18-tab ranked order, a decrease in numeric shade score represented an increase in tooth whiteness. Anticipating the effectiveness of long-term peroxide treatment, any subject who reached the lowest numeric shade (B1 or lower) before the end of treatment was withdrawn from active treatment by the investigator. For these subjects (< or = B1 before Month 6), the “last observation carried forward” method was used to compute shade improvement at subsequent visits. Tobit (censored regression) models were used to adjust for possible bias attributable to effectiveness-related early withdrawals.18,19 Treatment times were compared using analysis of covariance with the baseline shade as the covariant. Comparisons to baseline were one sided, using a 5% significance level.
Results
Age at baseline was 22 years to 58 years, with mean (standard deviation) of 37.7 (9.46) years (Table 2). Females accounted for 63.3%, and 25% of subjects reported a Southeast Asian origin. Tetracycline staining varied considerably, with 50% of participants presenting with moderate-to-severe tetracycline staining (levels II to IV). Shade scores ranged from 6 (corresponding to the “C1” tab) to 17 (“C4+”), with a baseline mean shade of 12 (corresponding to the “A3.5” tab). While tobacco use was uncommon (15% of the sample), 95% of participants consumed coffee, tea, or cola daily.
The treatment was effective overall in improving the shade of tetracycline-stained teeth, as evidenced by the significant (P < .05) improvement in shade after 1 to 2 months depending on treatment. After 1 month of treatment, on average, a four-shade improvement was observed. There was a statistically significant (P < .001) improvement in shade after 1 month (Table 3). All the subjects experienced incremental shade improvement with continued use after Month 1.
During the first 2 months of continual daily treatment, no participants reached or exceeded the minimal shade level. By Month 3, only two subjects reached “B1” or lighter and were withdrawn from treatment. After 6 months of daily treatment, 40% of subjects had not reached or exceeded this minimal shade cutoff. Use of the Tobit (censored regression) models, which adjusted for the biases in the treatment difference at Month 3, 4, 5, and 6 visits, yielded treatment differences from 1.3 to 0.6 shades (Table 4).
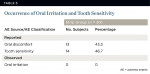
Mild and transient tooth sensitivity and oral irritation were the most common adverse events associated with treatment (Table 5). Findings were symptomatic, because no product-related clinical manifestations were observed at any monthly clinical examination. Occurrence of tooth sensitivity and oral discomfort was 43.3% to 46.7%. The onset was generally during the first month of treatment and transitory, resolving fully at treatment completion.
Four subjects discontinued treatment during the first 2 months; none of these dropouts were study related (one subject had a job relocated and the others did not return for scheduled appointments). No systemic or nonoral adverse events (nausea or others) relating to product usage were observed any time during the 6-month treatment period. No participants reported using any “rescue medication,” such as analgesics, for treatment-related sensitivity. Early withdrawal due to a product-related adverse event was not observed, and no subject reported modifying usage because of treatment-related irritation or sensitivity.
Discussion
This study evaluated clinical response following longer-term, daily use of H2O2-containing whitening strips. The target population consisted of individuals with tetracycline staining, because clinical research repeatedly has demonstrated the need for extended treatment for several months to achieve meaningful whitening with these cases.20 Subjects used their treatment throughout the 6-month period unless they reached an average VITA shade score of “B1” or lighter prior to the last evaluation. Those attaining this degree of whiteness were withdrawn from additional bleaching but were followed through the remainder of the trial for efficacy and safety outcomes.
In this research, the strip bleaching system was effective, yielding a highly significant (P < .001) and similar 10- to 11-mean shade improvement from baseline after 6 months of use. The 6.5% H2O2 strip system was used twice daily for 30 minutes with a 1-hour total daily contact time. In this study, the onset of shade improvement was early in the treatment and the change was greater at that time. Through Month 3, the group exhibited a statistically significant (P < .05) 1.3- to 3.2-shade improvement.
This study provides new evidence of an expanded safety in-use with strip-based tooth whitening. The bleaching system in this trial was well tolerated with daily use for a 6-month treatment period. No subjects discontinued treatment early due to product-related adverse events. The research provides the first evidence of the strip system in-use clinical safety with extended treatment. Previous studies have also indicated the use of 6% H2O2 on strips for 3 weeks resulted in no surface morphology or fracture susceptibility of teeth in vitro.21 The research, which expanded on an earlier report establishing clinical safety at concentrations up to 6.5% H2O2 on strips usage for up to 2 months of daily use, extended treatment duration to 6 months.11,22 Twice-daily use of the 6.5% H2O2 whitening strips was well tolerated for the 2-month period, with the principal side effects being transient tooth sensitivity and gingival irritation. This could be attributed to the higher peroxide concentration in the strip or other factors, including chance. Compared to the earlier 2-month findings, extended use resulted in only a few additional reports of sensitivity/irritation, with no increasing severity or persistence. Longer-term use through Month 6 showed no evidence of any cumulative toxicity.
Previous research has shown the safety and effectiveness of 10% carbamide peroxide used overnight in a tray-based system, in order to reduce tetracycline stains.10,23,24 This research suggests daytime use of whitening strips may be a viable option when indicated due to patient preference or other factors. For this study, 6.5% hydrogen peroxide strips were used. This is the lowest concentration available, as whitening strips are available with higher concentrations of hydrogen peroxide. Since good results were obtained with this low concentration of hydrogen peroxide, it is possible that a patient’s use of strips with a higher concentration of hydrogen peroxide would have even better results. For example, a higher concentration might shorten either the application time needed per day or the treatment time as a whole.
It should be noted that whitening strips on most patients cover the six anterior teeth; in some patients they extend to the first premolars. This may be a consideration when treating patients whose posterior teeth are visible when they smile. However, for most patients this does not seem to be a concern. In this study, for instance, patients were given the option to have trays after using the whitening strips, and none of the patients chose that option. Another potential drawback to using strips is that teeth need to be in a relatively ideal alignment. If teeth are crooked, crowded, or rotated it can effect whether the strip adequately covers the facial surfaces of the teeth. For these patients a custom tray system may be preferable.
Long-term clinical trials of this duration may be particularly relevant in establishing clinical safety associated with routine treatment as a research model that evaluates extreme (intentional or unintentional) use. Such research may be appropriate only with populations such as this one in which long-term treatment is common and/or necessary to achieve a desired endpoint, ethical and experimental controls are adequate to interpret outcomes, and rigorous and sufficient exit criteria are established to assure in-use safety. Under these conditions, longer-duration trials represent a “torture test” of sorts, offering additional insight into short-term clinical safety. This extended-duration research, in which whitening strips were professionally dispensed following clinical examination and used for 180 contact hours for 6 months to treat tetracycline stains, corroborates and extends the safety of strip-based tooth whitening as reported in earlier, shorter clinical trials.11,22,25,26
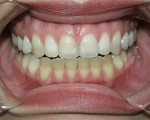
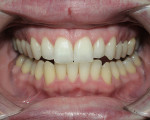
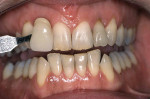
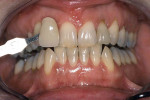
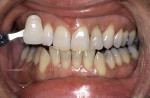
Tetracycline staining is complex and may necessitate extensive esthetic intervention. Vital bleaching with strip systems can yield an evident improvement in appearance in a few months (Figure 1). This noninvasive treatment may need to continue for an extended period. Some individuals will show a favorable response after 3 to 4 months, and treatment may be discontinued (Figure 2). Figure 3, Figure 4 and Figure 5 shows a progression of treatment through 6 months. The duration may be difficult to predict, given the variable clinical manifestations of tetracycline staining. Practitioners and patients should be advised that optimal bleaching for tetracycline staining may necessitate extended daily at-home treatment for 3 to 6 months. The costs, risks, and benefits of the various treatment options must be considered as part of overall patient management.
Conclusion
The 6.5% H2O2 bleaching strip provided significant tooth whitening of tetracycline stain. Daily use of strips for an extended 6-month period was generally well tolerated, with no subjects discontinuing treatment early due to mild tooth sensitivity or oral irritation.
Disclosure
This research was supported, in part, by The Procter & Gamble Company.
References
1. Arens D. The role of bleaching in esthetics. Dent Clin North Am. 1989;33(2):319-336.
2. Swachman H, Fekete E, Kulezychi L, Foley GE. The effect of long-term antibiotic therapy in patients with cystic fibrosis of the pancreas. Antibiot Annu. 1958-1959;(6):692-699.
3. Cohen S, Parkins FM. Bleaching tetracycline-stained vital teeth. Oral Surg Oral Med Oral Pathol. 1970;29(3):465-471.
4. Christensen GJ. Bleaching vital tetracycline stained teeth. Quintessence Int Dent Dig. 1978;9(6):13-19.
5. Mello HS. The mechanism of tetracycline staining in primary and permanent teeth. J Dent Child. 1967;34(6):478-487.
6. Moffitt JM, Cooley RO, Olsen NH, Hefferren JJ. Prediction of tetracycline-induced tooth discoloration. J Am Dent Assoc. 1974;88(3):547-552.
7. Mull MM. The tetracycline and the teeth. Dent Abstr. 1967;(12):346-350.
8. Parkins FM, Furnish G, Bernstein M. Minocycline use discolors teeth. J Am Dent Assoc. 1992;123(10):87-89.
9. Bowles WH, Bokmeyer TJ. Staining of adult teeth by minocycline: binding of minocycline by specific proteins. J Esthet Dent. 1997;9(1):30-34.
10. Haywood VB, Leonard RH. Six- and 12-month color stability after 6 months bleaching tetracycline teeth [abstract #2891]. J Dent Res. 1996;75(spec iss):379.
11. Kugel G, Aboushala A, Zhou X, Gerlach RW. Daily use of strips on tetracycline-stained teeth: comparative results after 2 months. Compend Contin Educ Dent. 2002;23(1A):29-34.
12. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-176.
13. Kugel G, Kastali S. Nontray whitening. Compend Contin Educ Dent. 2000;21(6):524-528.
14. Gerlach RW. Whitening paradigms 1 year later: introduction of a novel professional tooth-bleaching system. Compend Contin Educ Dent. 2002;23(1A):4-8.
15. Haywood VB. Frequently asked questions about bleaching. Compend Contin Educ Dent. 2003;24(4A):324-338.
16. Haywood VB, Leonard RH, Dickinson GL. Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent. 1997;9(1):13-19.
17. Boksman L, Jordan RE. Conservative treatment of the stained dentition: vital bleaching. Aust Dent J. 1983;28(2):67-72.
18. Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24-36.
19. Greene WH. Econometric Analysis. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 1993.
20. Leonard RH Jr. Long-term treatment results with nightguard vital bleaching. Compend Contin Educ Dent. 2003;24(4A):364-374.
21. White DJ, Kozak KM, Zoladz JR, et al. Effects of Crest Whitestrips bleaching on surface morphology and fracture susceptibility of teeth in vitro. J Clin Dent. 2003;14(4):82-87.
22. Li Y, Lee SS, Cartwright SL, Wilson AC. Comparison of clinical efficacy and safety of three professional at-home tooth whitening systems. Compend Contin Educ Dent. 2003;24(5):357-364.
23. Leonard RH Jr, Haywood VB, Caplan DJ, Tart ND. Nightguard vital bleaching of tetracycline-stained teeth: 90 months post treatment. J Esthet Restor Dent. 2003;15(3):142-152.
24. Matis BA, Wang Y, Jiang T, Eckert GJ. Extended at-home bleaching of tetracycline-stained teeth with different concentrations of carbamide peroxide. Quintessence Int. 2002;33(9):645-655.
25. Gerlach RW, Zhou X. Comparative clinical efficacy of two professional bleaching systems. Compend Contin Educ Dent. 2002;23(1A):35-41.
26. Karpinia KA, Magnusson I, Sagel PA, et al. Vital bleaching with two at-home professional systems. Am J Dent. 2002;15(spec no):13A-18A.
About the Authors
Gerard Kugel, DMD, MS, PhD
Professor
Department of Prosthodontics and Operative Dentistry
Associate Dean for Research
Tufts University School of Dental Medicine
Boston, Massachusetts
Robert W. Gerlach, DDS, MPH
Principal Scientist
The Procter & Gamble Company
Mason, Ohio
Ayman Aboushala, DMD, MS
Associate Clinical Professor
Tufts University School of Dental Medicine
Boston, Massachusetts
Susana Ferreira, DMD
Adjunct Assistant Professor
Tufts University School of Dental Medicine
Boston, Massachusetts
Britta Magnuson, DMD
Research Associate
Tufts University School of Dental Medicine
Boston, Massachusetts