In-Practice Evaluation of OraVerse® for the Reversal of Soft-Tissue Anesthesia After Dental Procedures
Timothy R. Saunders, DDS; Gregory Psaltis, DDS; John F. Weston, DDS; Rex R. Yanase, DDS; Siegfried S. Rogy, PhD; and Richard G. Ghalie, MD
Abstract
This study investigated the pattern of use, dentist evaluation, and patient assessment of OraVerse® (OV), a solution of phentolamine mesylate formulated for intraoral submucosal injection and used for the reversal of soft anesthesia after dental procedures. Participants were provided the drug for treatment of up to 10 patients each and agreed to complete a 26-item evaluation questionnaire at the end of the clinical assessment. Data were available from 51 dentists reporting on 390 patients 4 to 90 years of age. A total of 394 dental procedures were performed: 224 (57%) in the mandible and 170 (43%) in the maxilla. Local anesthetics most frequently used were lidocaine/epinephrine (66.4%) and articaine/epinephrine (23.6%). In 81.5% of cases, OV was administered after restorative procedures. This OV dose was given as one-half, one, and two cartridges in 11.8%, 76.7%, and 10.3% of patients, respectively. An adverse reaction at the injection site was reported in 19 patients (4.9%). The median times to return to normal after injection were 60 minutes for lip sensation, 57.5 minutes for tongue sensation, and 60 minutes for oral function. Patients reported reduced duration of oral numbness (92%) and improved dental experiences (84%) after use. A total of 83% of patients said they would recommend the medication to others and 79% said they would opt for OV in the future. Dentists reported that the medication addressed an existing need (86%), met expectations (82%), was a practice differentiator (55%) and a practice builder (45%), and improved scheduling (29%). In this in-practice clinical evaluation, times to return to normal oral sensation and function after OV administration were consistent with those reported in randomized clinical studies. Both patient and dentist satisfaction rates were high.
Local anesthetics block pain sensation by interfering with the propagation of peripheral nerve impulses. The effects of local anesthetics vary according to onset, depth, and duration of anesthesia.1 Local anesthetic solutions usually contain a vasoconstrictor to improve the depth and duration of the anesthesia and to reduce bleeding in the operative field.2,3 By counteracting the localized vasodilator effects of local anesthetics on subcutaneous and submucosal vessels, vasoconstrictors enhance neuronal uptake by increasing the anesthetic residence time in tissues, and reduce the risk of anesthetic toxicity by decreasing the rate of systemic absorption from the injection site. Local anesthetics containing a vasoconstrictor account for more than 90% of total anesthetic use in dentistry.4,5
Local anesthesia eradicates pain from certain hard tissues (eg, tooth, bone) and soft tissues (eg, gingiva) as appropriate for the planned dental procedure. Depending on the injection site, method of administration, and dose, this results in pulpal and periodontal anesthesia, as well as anesthesia in soft tissue such as lip, tongue, and cheek. Anesthesia of soft tissues is not needed for many dental procedures.6 Furthermore, soft-tissue anesthesia usually lasts 3 to 5 hours, which greatly exceeds the time needed for routine dental and periodontal maintenance procedures.7 This prolonged and unnecessary soft-tissue anesthesia is described by many patients as uncomfortable and inconvenient because it interferes with daily activities. In addition, prolonged soft-tissue anesthesia has been associated with accidental, self-inflicted soft-tissue injury, particularly in children.8-10
Phentolamine mesylate, a competitive antagonist of α-adrenergic receptors, is indicated for several medical conditions (eg, pheochromocytoma, prevention of dermal necrosis after accidental injection of epinephrine) where it is administered parenterally at doses from 1 mg (children) to 10 mg (adults). OraVerse® (Septodont, www.septodontusa.com) is a solution of phentolamine mesylate formulated for intraoral submucosal injection. It is available in standard dental cartridges containing 0.4 mg of phentolamine mesylate in a 1.7-mL solution. After binding to α-adrenergic receptors present on vascular smooth muscles, phentolamine induces rapid and transient vasodilatation. When pulpal anesthesia is no longer needed at the end of a dental procedure, OV injected at the same injection site as the local anesthetic induces localized vasodilatation and increases the clearance of the local anesthetic.11 The presumed mechanism by which OV accelerates the reversal of local anesthesia is via faster clearance of the anesthetic from local tissues and nerves.12
In two double-blind controlled studies involving 484 adolescents and adults undergoing routine dental and periodontal maintenance procedures following local anesthesia with one of four anesthetics containing a vasoconstrictor, OV significantly accelerated the median time to return to normal sensation of the upper lip by 82.5 minutes (50 minutes vs 132.5 minutes), lower lip by 85 minutes (70 minutes vs 155 minutes), and tongue by 65 minutes (60 minutes vs 125 minutes) compared to the control group.13 Furthermore, OV significantly accelerated the median time to return to normal function by 60 minutes (60 minutes vs 120 minutes) after mandibular procedures and 45 minutes (60 minutes vs 105 minutes) after maxillary procedures.13 In a double-blind controlled pediatric study, OV significantly hastened the median time to normal sensation of the lip by 75 minutes (60 minutes vs 135 minutes) and tongue by 67.5 minutes (45 minutes vs 112.5 minutes) compared to the control group in 115 trainable patients age 6 to 11 years who underwent local anesthesia with lidocaine/epinephrine.14 In these studies, adverse reactions with OV were mild or moderate, as well as transient. These reactions had an incidence comparable to the control groups, which received a simulated injection. In the randomized studies, injection site pain was the most common adverse reaction, with comparable frequency reported with OV and the control groups (5% vs 4%).
The US launch of OV was February 2009. The authors conducted this study to evaluate OV use in clinical practice and compare outcomes observed in real-world conditions to those reported in the controlled clinical studies. The main objectives were to investigate the pattern of OV use by clinicians, determine how patients perceive OV effects, and assess how dentists evaluate the introduction of OV to their practices.
Materials and Methods
Dentists were selected if they had not used OV previously and agreed to complete a 26-item questionnaire at the end of their clinical assessment. Thirteen questions captured data on patient age and weight, location (mandibular or maxillary) and type of procedure (restorative, periodontal, or other), type and dose of local anesthetic, OV dose, and local tolerability to the injections of OV and the local anesthetic. Two questions evaluated the time to return to normal lip and tongue sensation as self-assessed by patients by tapping their lip/tongue with their index fingers, and one question gauged time to return to normal oral function (smiling, speaking, drooling control) as reported to the healthcare professional while in the clinic or during a follow-up phone call (Table 1). Four questions evaluated the patient assessment of OV, and six questions analyzed the dentists’ assessments of OV usage on their practices.
Each participant received one carton of 10 OV cartridges to be used in accordance with the Food and Drug Administration (FDA)-approved prescribing information in up to 10 patients. OV was to be administered at the end of the dental procedure, when pulpal anesthesia was no longer needed, using the same injection technique (infiltration or block) and at the same injection site as the local anesthetic. OV was to be dosed on a 1:1 cartridge ratio to the local anesthetic/vasoconstrictor administered, with a maximum dose of one-half cartridge (0.2 mg) in children at least 6 years old and 33 lbs to 66 lbs, one cartridge (0.4 mg) in children 6 to 11 years old and at least 66 lbs, and two cartridges (0.8 mg) in patients 12 years or older.
Because not all participants were likely to return their evaluation forms, the authors planned to enroll 100 participants in general and pediatric practices, expecting to collect data on approximately 400 patients, a sample size comparable with the number of dental patients given OV in the controlled studies.13-15 Data were analyzed using standard descriptive statistics and 95% confidence interval (CI) calculated when appropriate.
Results
Between April and December 2009, dentists from 14 states enrolled in the study and then received OV and the questionnaire form. A total of 51 dentists returned the completed questionnaires within a median of 86 days (range 7 to 304 days) after receiving OV. Eleven of the 51 respondents (22%) had patients younger than 18 years. Final data analysis was performed in March 2010, and interim results were presented elsewhere.16
Information is available on 390 patients, between 4 and 90 years. Respondents treated a median of eight patients (range one to 10). A total of 394 dental procedures were performed: 224 (57%) in the mandible and 170 (43%) in the maxilla. Treatment characteristics are shown in Table 2. The most frequent type of procedures performed was restorative (81.5%)—a single cartridge of OV was administered in 76.7% of patients. The most frequently administered local anesthetics were lidocaine/epinephrine (66.4%) and articaine/epinephrine (23.6%). Injection site reactions (erythema, pain, edema, and/or hemorrhage) were reported in 32 patients (8.2%, 95% CI 5.7% to 11.4%) after the local anesthetic injection and 19 patients (4.9%, 95% CI 3.0% to 7.5%) after OV injection.
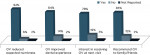
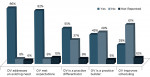
The median time from OV injection to recovery of normal sensation was 60 minutes (5 minutes to 210 minutes) for the lip and 57.5 minutes (5 minutes to 225 minutes) for the tongue. The median time from OV injection to return to normal oral function was 60 minutes (5 minutes to 240 minutes). Patients’ evaluation of their experiences with OV is presented in Figure 1. Overall, 92% of patients reported OV reduced the expected duration of soft-tissue anesthesia, 84% said OV improved their dental experiences, 83% said they would recommend OV to family and friends, and 78% expressed an interest in receiving OV after local anesthesia. Dentists’ assessments of the effect of OV are presented in Figure 2. Overall, 86% of the dentists reported OV addresses an existing need and 82% reported OV met their expectations. Implementation of OV was considered a practice differentiator by 55% of dentists and a practice builder by 45%. Also, 29% of dentists noted OV would allow more flexibility in scheduling appointments because patients are less concerned about lingering soft-tissue anesthesia affecting daily activities.
Discussion
This is the largest study to date evaluating the use of OV in clinical practice. With data collected from 51 dentists and 390 patients, this evaluation complements the efficacy and safety profile available from 399 dental patients treated with OV in randomized clinical studies.13-15 While the clinical studies controlled the criteria for patient enrollment, the location of dental procedures, and the type of local anesthetic with vasoconstrictor administered, this evaluation required only that patients receive treatment according to the FDA-approved prescribing information for OV. Therefore, this evaluation represents real-world experience with the use of OV.
Overall, there were no substantial differences in OV usage in clinical practice compared to the clinical studies. Most patients who received OV had restorative procedures; 81.5% of patients in this study, compared with 69.9% in the OV group in the adult/adolescent Phase 3 studies.13 One cartridge of OV was administered in 76.7% of patients in this study compared with 85% in the adult/adolescent Phase 3 studies.13 Lidocaine/epinephrine was the predominant local anesthetic used in this study (66.4% of patients), consistent with another report on the usage of local anesthetics in the United States.17
Times to return to normal soft-tissue sensation and oral function were similar to those reported in the randomized adult/adolescent Phase 3 studies.13 More specifically, the median time to normal lip sensation was 60 minutes in this study compared with 50 minutes for upper lip and 70 minutes for lower lip in the Phase 3 studies, and the median times to normal tongue sensation were 57.5 minutes and 60 minutes in this study and the Phase 3 studies, respectively.13 Similarly, the median time to return to normal oral function was 60 minutes in this and the Phase 3 studies. The frequency of local reactions at the site of OV injections was 4.9% in this study, comparable to the frequency of injection site pain/reaction reported in the adult/adolescent studies (6.7%) and the pediatric study (6.2%).13,14 These findings confirm that, when OV is used in the general population, its efficacy and safety profile is comparable to that observed in a controlled setting.
Patient assessment of the drug was favorable, with most patients reporting OV improved their dental experiences and would recommend the drug to family and friends. Clinicians noted the clinical benefit, with most dentists reporting it addresses an unmet clinical need and meets expectation. Furthermore, several practitioners indicated that the implementation of OV would positively impact their practice differentiation and growth. Of note, these results were similar to those reported in another independent in-practice evaluation of OV involving 22 clinicians and 104 patients.18
Conclusion and Clinical Implications
Lingering soft-tissue anesthesia is often an unnecessary and undesired secondary effect of dental anesthesia. Until recently, there has been no approved method to reduce the duration of soft-tissue anesthesia and the associated functional deficits after the administration of a local anesthetic with a vasoconstrictor. The addition of OV to the dental pharmaceutical armamentarium fulfills this unmet clinical need. The times to return to normal lip and tongue sensation and oral function when OV is used in clinical practice were consistent with those reported in controlled clinical studies.
In the authors’ opinions, patients who would benefit the most from OV are those undergoing dental procedures in which postprocedural pain is not expected, including restorative procedures such as cavity preparation; crown preparation and placement; and inlays, onlays, and veneers placement, as well as nonsurgical periodontal scaling and root planing. OV can be readily introduced into clinical practice because the method and equipment used for its administration are identical to those employed for local anesthetics. Based on the favorable assessment of both patients and dentists, OV is a valuable addition to clinicians’ pharmaceutical armamentarium.
Disclosure
Funding for this study was provided by Novalar Pharmaceuticals. Dr. Rogy and Dr. Ghalie are Novalar employees.
Acknowledgments
The authors wish to thank the clinicians who contributed to this study and Ms. Var Galpchian, who managed the study.
References
1. Yagiela JA. Local anesthetics. In: Yagiela JA, Neidle EA, Dowd FJ, eds. Pharmacology and Therapeutics for Dentistry. 5th ed. St. Louis, MO: Mosby; 2004:251-270.
2. Malamed SF. Pharmacology of vasoconstrictors. In: Malamed SF, ed. Handbook of Local Anesthesia. 5th ed. St. Louis, MO: Mosby; 2004:41-53.
3. Becker ED, Reed KL. Essentials of local anesthetic pharmacology. Anesth Prog. 2006;53(3):98-109.
4. Gaffen AS, Haas DA. Survey of local anesthetic use by Ontario dentists. J Can Dent Assoc. 2009;75(9):649.
5. Corbett IP, Ramacciato JC, Groppo FC, Meechan JG. A survey of local anaesthetic use among general dental practitioners in the UK attending postgraduate courses on pain control. Br Dent J. 2005;199(12):784-787.
6. Hersh EV, Hermann DG, Lamp CJ, et al. Assessing the duration of mandibular soft tissue anesthesia. J Am Dent Assoc. 1995;126(11):1531-1536.
7. Malamed SF. Clinical action of specific agents. In: Malamed SF, ed. Handbook of Local Anesthesia. 5th ed. St. Louis, MO: Mosby; 2004:79.
8. Adewumi A, Hall M, Guelmann M, Riley J. The incidence of adverse reactions following 4% septocaine (articaine) in children. Pediatr Dent. 2008;30(5):424-428.
9. College C, Feigal R, Wandera A, Strange M. Bilateral versus unilateral mandibular block anesthesia in a pediatric population. Pediatr Dent. 2000;22(6):453-457.
10. Akram A, Kerr RMF, Mclennan AS. Amputation of lower left lip following dental local anaesthetic. Oral Surg. 2008;1(2):111-113.
11. Koss MC. Differential neural activation of vascular alpha-adrenoceptors in oral tissues of cats. Eur J Pharmacol. 2002;440(1):53-59.
12. Moore PA, Hersh EV, Papas AS, et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesth Prog. 2008;55(2):40-48.
13. Hersh EV, Moore PA, Papas AS, et al. Soft Tissue Anesthesia Recovery Group. Reversal of soft-tissue local anesthesia with phentolamine mesylate in adolescents and adults. J Am Dent Assoc. 2008;139(8):1080-1093.
14. Tavares M, Goodson JM, Studen-Pavlovich D, et al. Soft Tissue Anesthesia Reversal Group. Reversal of soft-tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139(8):1095-1104.
15. Laviola M, McGavin SK, Freer GA, et al. Randomized study of phentolamine mesylate for reversal of local anesthesia. J Dent Res. 2008;87(7):635-639.
16. Saunders TR, Psaltis G, Weston JF, et al. Survey of OraVerse in-practice use for soft tissue anesthesia reversal. International Journal of Dental Research [abstract 718]. 2010.
17. Moore PA, Nahouraii HS, Zovko J, Wisniewski SR. Dental therapeutic practice patterns in the U.S. I. Anesthesia and sedation. Gen Dent. 2006;54(2):92-98.
18. OraVerse–The first and only local anesthesia reversal agent. Clinician’s Report. 2010;3(3).
About the Authors
Timothy R. Saunders, DDS
Professor of Clinical Dentistry
Department of Restorative Sciences
Herman Ostrow School of Dentistry of the University of Southern California
Los Angeles, California
Gregory Psaltis, DDS
Private Practice
Olympia, Washington
John F. Weston, DDS
Director, Scripps Center for Dental Care
San Diego, California
Rex R. Yanase, DDS
Private Practice
Torrance, California
Siegfried S. Rogy, PhD
Senior Director, Clinical Operations
Novalar Pharmaceuticals, Inc
San Diego, California
Richard G. Ghalie, MD
Chief Medical Officer
Novalar Pharmaceuticals, Inc
San Diego, California