Alveolar Ridge Augmentation: Comparison of Two Socket Graft Materials in Implant Cases
Len Tolstunov, DDS; and Jibin Chi, MD, MBA, MBI
Abstract
With the variety of bone grafting materials available and their use around both natural teeth and dental implants, clinicians need to understand not only basic bone biology but also characteristics of different bone grafting materials to make a proper choice when selecting a material for alveolar bone augmentation and implant treatment. The grafting materials used in the maxillofacial region include autogenous bone, allografts, xenografts, alloplastic or synthetic products, and composite grafts (combination of different materials). This case report describes two frequenly used bone graft materials for socket preservation immediately after extraction: Puros® (Zimmer Dental Inc, www.zimmerdental.com) (allogeneic) and Bio-Oss® (Osteohealth Co, www.osteohealth.comwww.osteohealth.com) (xenogeneic). In the case presented, the authors perform biologic, clinical, radiologic, and histologic analysis of the two grafting materials by placing them side-by-side in the same patient during implant reconstruction. Implant-related phases of bone augmentation are proposed, and an overview of bone grafting materials, specifically Bio-Oss and Puros, is presented.
A variety of bone grafting materials and membranes have been introduced over the years for alveolar bone augmentation and ridge preservation in proximity of natural teeth. With advances in oral implantology in the past 30 years, old and new bone grafting materials have also been used for alveolar bone preservation, augmentation, and regeneration around dental implants. Successful grafting has been accomplished with particulate autogenous, allogeneic, xenogeneic, and synthetic bone grafts and bone graft substitutes; barrier membranes; autogenous and allogeneic block grafts; and composite grafts;1-8 and through the use of osteoinductive effects of bone-morphogenic protein (BMP-2)9 and others. Recombinant human BMP (RhBMP)-2 has been proven effective in inducing bone formation. Recently, the US Food and Drug Administration approved INFUSE® bone graft (rhBMP-2/ACS)10,11 by Medtronic Inc (www.medtronic.com) for alveolar bone grafting and subantral augmentation procedures. This new material seems to have a significant potential for the de novo bone formation of bone quality similar to natural bone. GEM 21S® (Osteohealth) is another innovative synthetic regenerative material that is a combination of a bioactive protein (purified recombinant human platelet-derived growth factor, rhPDGF-BB) and a biocompatible osteoconductive matrix (beta-tricalcium phosphate, β-TCP). It shows significant promise in the regeneration of deficient bone in periodontal defects around natural teeth and defects associated with peri-implantitis around implants.
The primary goals of many prosthetic-driven surgical bone grafting techniques are to preserve the socket and alveolar ridge anatomy and create a strong foundation for osseointegrated implants that are to be restored to normal function, esthetics, phonetics, and comfort. Success in ridge preservation (soft and hard tissue) is extremely important in optimizing future implant function.
Table 1). Osteogenesis is bone formation directly from the transplant's or host's living bone cells (osteoblasts and osteocytes). This process occurs mostly with autogenous grafts or tissue engineering. Osteoinduction is an indirect bone-induction method where the introduced graft has the potential to induce or stimulate bone growth by activating the mesenchymal (stem) cells in adjacent bone to differentiate and proliferate into bone-forming cells (osteoblasts) and, eventually, osteocytes. Osteoinductivity is linked to BMPs, which must be present within the organic matrix of bone. This process occurs with autogenous grafts, demineralized freeze-dried human bone allografts (DFDBA), and the rhBMPs, which are members of the transforming growth factor-β superfamily. Osteoconduction is bone formation initiated from the surrounding viable bone cells, while the bone graft acts as a scaffold to allow the deposition of newly formed bone and, through the mechanism of creeping substitution, can be gradually replaced with the host bone. This process occurs mostly with xenogeneic and synthetic grafts.
The most common bone grafting materials are osteoconductive in nature (some can be weakly osteoinductive) and are widely regarded as bone grafting expanders rather than true bone grafting replacements. Mineralized allograft, xenograft, and alloplastic materials belong to this category. Compositions of different bone grafting materials ("graft salads") also are commonly used to optimize bone regenerative potentials. A clinician may choose to use an osteoconductive material like an anorganic bovine bone and osteoinductive material like DFDBA to benefit from both mechanisms of bone formation. The choice of these composite grafts is based largely on the surgeon's preference and is not always scientifically based.
Implant-Related Phases of Bone Augmentation
Chronologically, implant-related alveolar guided-bone regeneration (GBR) can be divided into three phases relative to the time of implant insertion (Table 2).
Phase 1: Preimplant Phase of Bone Augmentation
This preparatory hard-tissue procedure is used in indicated cases before dental implantation to preserve or form the foundation necessary for the future placement of an endosseous implant. Examples of this grafting approach include socket bone grafting after extraction of a failed tooth, monocortical block bone graft in the area of horizontal alveolar bone resorption, and direct (transalveolar) sinus lift/bone graft for a severe vertical deficiency of the alveolar ridge in the posterior maxilla. Implant placement is deferred from 4 to 9 months (depending on the site and type of bone augmentation) to allow sufficient time for the bone healing before endosseous implant placement.
Phase 2: Implant Phase of Bone Augmentation
This procedure is used at the time of implant placement. Examples include cases of immediate implantation after the extraction of a failed tooth simultaneously with bone grafting for the peri-implant space, an indirect (crestal) osteotome sinus lift/bone graft with immediate implantation, and a ridge-splitting/expansion procedure with simultaneous implant insertion.
Phase 3: Postimplant Phase of Bone Augmentation
GBR techniques can be performed after completion of the restorative stage, or at any time during the process of osseointegration (the first year after implant insertion and beyond). This secondary grafting is often directed toward implant salvation in cases of peri-implantitis or other implant-related complications. This phase follows principles of grafting that are similar to grafting around failing natural teeth.
Some cases need multiple rounds of bone grafting before and during an implant reconstruction. An example of this combined approach is an extraction of a tooth in the posterior maxilla and direct subantral augmentation with bone graft of the subantral space (Phase 1), followed by an implant placement in the posterior maxilla 6 months later and an additional grafting for the correction of the alveolar ridge deficiency (Phase 2). In other cases, there may be a need for secondary grafting years later if the implant has lost bone because of traumatic occlusion or peri-implantitis (Phase 3). These occasionally encountered clinical situations can be described as a multiphasic mode of alveolar implant-related bone reconstruction.
The presented case report offers a clinical look at two osteoconductive bone grafting materials—Puros® (allogeneic) and Bio-Oss® (xenogeneic)—used on the same patient during implant reconstruction. The results were evaluated and compared clinically, radiographically, and histologically.
Case Report
A 56-year-old woman was referred for a consultation regarding her failing upper first molars. The patient had occasional discomfort from both teeth (Nos. 3 and 14) and difficulty chewing on both sides. Root canal treatment had been performed on her upper right first molar twice during the previous 2 years. The patient had taken several courses of penicillin to control periodic infections associated with both molars. After endodontic and periodontal evaluations, an implant reconstruction was suggested to the patient. The patient did not have medical contraindications for implant treatment. Her dental history was positive, with only mild to moderate bruxism that was controlled with a nightguard.
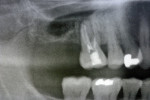
On examination, the patient had an overall stable dentition with Class I malocclusion, positive protrusive clearance, group function occlusion bilaterally, and wear facets present on the posterior teeth. Both maxillary first molars had mobility 2+ with periodontal bone loss, gingival recession, and root exposure. Widening of the periodontal ligament (teeth Nos. 3 and 14) and proximity of roots to the maxillary sinus floor bilaterally were evident on the initial panoramic radiograph (Figure 1 and Figure 2).
Postextraction treatment plan options were discussed with the patient during an initial evaluation. Two main options were an upper partial denture or fixed implant-supported restorations. The patient elected implant treatment for both segments. Placement of two implants on each side to adequately restore the function for this patient with bruxism was contemplated. However, because of the presence of chronic periodontal and endodontal infection around both molars, the decision was made to follow a staged approach and remove both teeth first with socket bone grafting (Phase 1), followed by delayed implant insertion. Two different socket-grafting materials were used in the process of implant treatment for this patient.
Study Design
The study design consisted of a comparative analysis of two different bone grafts (Bio-Oss and Puros), and was planned to consist of clinical, radiographic, and histological examination of the alveolar bone at different stages of implant reconstruction. Periodic clinical and radiographic assessment of bone and soft-tissue healing was done, and bone level was measured. A histological examination of bone biopsies and back-scattered scanning electron micrographs (BSEM) were done at two different laboratories for comparison at 3 months after the socket bone grafting (before Implant Surgical Stage 1) and at the time of Implant Surgical Stage 2. Puros allograft was used on the right maxilla (site No. 3), and Bio-Oss xenograft was used on the left maxilla (site No. 14).
Surgical Preimplant Stage (Phase I)
The patient was scheduled for Phase 1 of implant treatment: pre-implant alveolar bone augmentation simultaneously with extraction of teeth Nos. 3 and 14 (Table 2). The procedure was based on Sclar's Bio-Col technique for socket preservation through GBR principles.12,13 The Bio-Col technique involves Bio-Oss bovine mineral that is packed into an extraction socket, followed by CollaTape® or CollaPlug® (Zimmer Dental) that is placed on top of the particulate graft, and finally, Iso-Dent™ adhesive (Ellman International, Inc, www.ellman.com) to seal and protect the grafted socket from the oral environment during the initial healing. The buccal flaps are not advanced, and the secondary (rather than primary) healing is emphasized. This is important to preserve the mucogingival junction position and the overall soft-tissue profile in the grafted site.
To compare different grafting materials in the same patient, the classic Bio-Col technique was used on the left side (tooth No.14) with the Bio-Oss graft, and a modified Bio-Col technique was used on the right side (tooth No. 3) with Puros grafting material. In both cases, CollaTape membrane and Iso-Dent adhesive were used to cover and seal the sockets.
The procedure was done under intravenous sedation per patient request. Teeth Nos. 3 and 14 were extracted atraumatically with the help of periotome and universal upper extraction forceps. The sockets of both teeth were debrided and examined for the lack of fenestration, dehiscence, and sinus communication. Both sockets appeared to have all four walls intact and no sinus perforations. The papillae in between teeth Nos. 3 and 4, as well as teeth Nos. 13 and 14, were preserved, and no vertical incisions were made.
At site No. 3, Puros allograft cancellous particles were packed into the socket according to instructions to the level of the surrounding crestal bone (0.25-mm to 1-mm particles, 0.5 cc). At site No. 14, cancellous Bio-Oss particles (0.25-mm to 1-mm particles, 0.5 cc) were loosely packed into the socket to the crestal level. CollaTape membrane 1 cm x 1 cm was placed on top of each socket to isolate the graft and was slightly tagged underneath the gingival soft tissue to fully cover the grafting material inside the sockets. Then, 4-0 chromic gut was used to approximate the edges of the surrounding soft tissue. Iso-Dent adhesive (according to the Bio-Col technique) was then added on top to seal both sockets from the oral environment.
The patient tolerated the sedation and the procedure well. She reported slightly more discomfort on the left side at the 1-week follow-up appointment. On examination at the 1-week postoperative appointment, the left (Bio-Oss) side had moderate erythema and swelling, the right (Puros) side had a good healing-tissue response and an uneventful recovery period (Table 3). At the 1-month follow-up appointment, the patient was asymptomatic and the examination showed well-healed sockets on both sides.
To adequately rehabilitate the patient's function and provide sufficient bilateral occlusion, the decision was made to attempt to restore her posterior mastication by extending occlusion to the level of the second molars. The measurements showed that it would require about 15 mm of alveolar length in the posterior maxilla bilaterally, which had been lost after extraction of the first molars. Placement of two implants on each side (site Nos. 2, 3, 14, and 15) was suggested and accepted by the patient for a fully functional, rehabilitated posterior occlusion.
Evaluation of the Alveolar Bone Augmentation
Preimplant alveolar bone level was assessed clinically, radiographically, and histologically 3 months after socket grafting.
Preimplant Clinical Assessment
Clinically at 3 months after the GBR, three measurements were taken to determine the surgical-prosthetic space (SPS) needed for successful implant reconstruction of the patient: (1) alveolar length, from the cemento-enamel junction of the adjacent tooth (second premolar) distally to accommodate two root-form implants; (2) alveolar width, the width of the alveolar crest in the upper first molar region of the healed sockets on both sides; and (3) interocclusal distance or vertical clearance, the interocclusal space from the occlusal surface of the mandibular first molar to the maxillary alveolar crest on full closure. These three postgraft and preimplant measurements on both sides in the area of the first molar were the same and equal to 15 mm x 9 mm x 7 mm (alveolar length x width x occlusal clearance) (Table 3). An adequate band of keratinized gingiva (3 mm) was present on both sides extending into the normal vestibule of a good depth bilaterally. The gingiva on both sides was of normal color without evidence of inflammation.
Preimplant Radiographic Assessment
Preimplant panoramic examination evaluated the possibility to distinguish cortical outlines of the sockets 3 months after the GBR procedure with two different bone grafts (ie, radiographic healing). The Puros (site No. 3) graft had integrated completely with no radiographically visible outline of the socket. The Bio-Oss (site No. 14) graft had healed with a radiographically visible outline of the socket. It appeared at this stage that Bio-Oss had not fully matured relative to the host bone, but particles of Puros had. Radiographically, the volume of both sockets had been preserved without noticeable bone collapse.
Implant Surgical Stage 1
Three months after the GBR procedure, the patient was scheduled for implant Surgical Stage 1, placement of two endosseous implants on each side. Diagnostic models were analyzed for implant position and a surgical stent was fabricated and made ready for the procedure.
The procedure was done again under intravenous sedation per patient request. Small buccal full-thickness flaps with distal-releasing incisions were raised bilaterally. Before the implant osteotomies were made the bone was evaluated clinically on both sides. The bone on the right side (Puros) had a smooth appearance, without porosities or fibrous tissue, and appeared uniformly dense. It was impossible to distinguish the particles of Puros bone graft from the host bone. The bone on the left side (Bio-Oss) had multiple porosities and some fibrous tissue entrapment that had to be cleaned out, and appeared less uniform. The particles of Bio-Oss bone graft could be easily seen in small amounts and macroscopically appeared to be incorporated partially into the host bone.
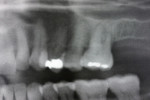
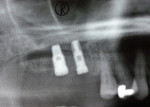
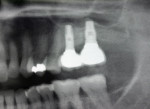
Two external hex implants (Restore®, Lifecore Biomedical, LLC, www.lifecore.com) were inserted on each side into the alveolar bone (site Nos. 2, 3, 14, and 15) with help of a surgical stent. The implants for site Nos. 3 and 14 (first molar position) were 4 mm in diameter and 11.5 mm in length; implants for site Nos. 2 and 15 (second molar position) were 5 mm in diameter and 10 mm in length. These endosseous root-form implants were inserted according to the standard protocol at the distance of 5 mm and 13 mm from the adjacent second premolars bilaterally and into the alveolar bone at the base of the maxillary sinus without the need for subantral augmentation. Implant cover screws were placed, and the flaps were closed primarily with 4-0 chromic gut sutures. Postoperative panoramic examination showed good position of all four implants in the posterior maxilla bilaterally (Figure 3 and Figure 4).
Preimplant Histological Assessment
In addition to preoperative clinical and radiographic evaluation of the socket graft healing, a histological assessment also was done to estimate the degree of graft integration into the host bone and resulting bone quality. A bone biopsy was done at Surgical Stage 1 at the time of implant insertion. A small wedge of bone was harvested from both implant sites and sent for histological examination to the oral pathology laboratories at University of California at San Francisco (UCSF) in the United States and Progentix, Bilthoven in the Netherlands.
The UCSF laboratory was asked to microscopically evaluate bone graft healing and vascularization. Bone graft healing was assessed based on the incorporation of the grafted material into the host bone using a scale from 0% to 100%, where 0% was bone that was isolated from the host bone and did not show any potential for integration, and 100% was the grafted bone that was fully incorporated into the surrounding bone and could not be distinguished from the host bone. The degree of vascularization was difficult to quantify and it was suggested to the oral pathologist to measure it on the scale from 0 to 10, where 0 was "dead-like" or sequestrated-like bone without any blood supply and 10 was very richly vascularised bone with plenty of capillaries.
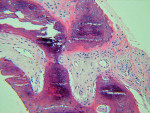
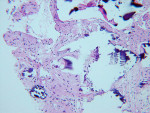
The UCSF laboratory results at 3 months after the bone graft for the Puros side was positive for sclerotic lamellar and woven bone with inflammation, no foreign material seen, 100% bone healing, and vascularisation at scale 5 (Figure 5). Puros was integrated completely into the newly formed bone, and it was impossible to distinguish any residual Puros granules. The UCSF laboratory results for the Bio-Oss side were positive for sclerotic bone with inflammation and foreign material, 20% bone healing, with vascularization at scale 5 (Figure 6 ). Bio-Oss showed slow and partial integration with easily distinguishable Bio-Oss granules at the time of investigation. The Progentix laboratory also conducted the same histological evaluation, and found less integration of Bio-Oss into the surrounding host bone at the same time point (Figure 7).
At 1 week after surgery, a small dehiscence of the flaps and inflammation on the Bio-Oss (site Nos. 14 and 15) side was noticed. It slowly increased as time progressed. The Puros (site Nos. 2 and 3) side showed normal healing with primary closure. The patient was rinsing with chlorhexidine 0.12% for 2 weeks postsurgery. At 5 months, the Bio-Oss side had healed with the implant cover screws partially exposed, and the Puros side had healed with submerged (subgingival) implant position. One month after Surgical Stage 1, the patient started to wear the relined transitional upper partial denture retained by clasps on her upper second premolars.
Implant Surgical Stage 2
Implant Surgical Stage 2 was performed 6 months after Surgical Stage 1, which was 9 months after socket bone grafting. Under local anesthesia, both implants were exposed and found to be rigid and fully integrated into the bone. Four temporary healing abutments (two on each side) were placed. Because of the exposure of the implants on the Bio-Oss side, flaps and sutures were not required. On the Puros side, flaps were closed around healing abutments with 4-0 chromic gut.
At Surgical Stage 2, the second biopsies of the alveolar bone (small amount of bone at the alveolar crest just adjacent to the first molar implants) were taken to see the changes in the grafted bone bilaterally. It was sent to the Progentix laboratory for the second histological evaluation.
Postimplant Histological Assessment
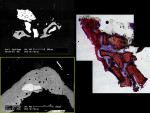
BSEM and histological evaluation of Puros and Bio-Oss biopsies at Progentix laboratory 9 months after socket bone grafting and 6 months after implant insertion showed similar levels of new bone formation in both Puros and Bio-Oss sites. However, in the Bio-Oss biopsy, granules remained at the site, which were surrounded mostly by fibrous tissue and isolated from the newly formed bone (Figure 8); in the Puros biopsy, the newly formed bone was distributed evenly and there were no signs of remaining granules (Figure 9).
Restorative Implant Stage
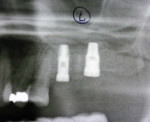
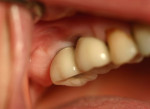
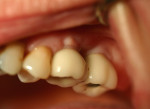
Two months after implant Surgical Stage 2, the patient was referred to the general dental office for the restorative stage. Two splinted, screw-retained, implant-supported porcelain-fused-to-metal (PFM) bridges were constructed and cemented to custom-permanent implant-supported abutments (Figure 10 and Figure 11). The patient had full masticatory function, protective group function occlusion bilaterally, good gingival architecture around the implant-supported crowns, and good esthetics. The general practitioner did mild occlusal reduction on the mandibular second-molar crowns to improve the interocclusal molar relationship with the maxillary implant crowns. A final panoramic radiographic evaluation 3 months after the delivery of the final prosthesis showed stable alveolar bony foundations around the implant fixtures, adequate to support implant restorations (Figure 12 and Figure 13 ). Bone levels were at the first fixture thread for all four implants.
After the procedure was completed, the patient received instructions for function and hygiene. The patient's expectations—function, esthetics, phonetics, and comfort—were met, and she was satisfied with the final result. The patient continued to wear a night guard to protect her dentition from the detrimental forces of bruxism. Comparison of both grafted materials during the implant reconstruction in this case is summarized in Table 3.
Discussion
Bio-Oss
Bio-Oss is a natural, porous bone mineral matrix that is produced by removal of all organic components from bovine bone. Bio-Oss, by its characteristics, is physically and chemically comparable with the mineralized matrix of human bone. It is sterilized by gamma irradiation. The trabecular architecture and different sizes of interconnecting pores of Bio-Oss promotes bone ingrowth and formation at the site of placement. Bio-Oss particulate material (granule size = 0.25 mm to 2 mm) can be used alone or as a mixture with autogenous bone. In the literature, Bio-Oss is often presented as a grafting material with an ideal osteoconductive scaffold that can be used for socket grafting (with or without immediately inserted implants) and sinus augmentation.12-18 Berglundh and Lindhe19 and Block and Sclar20 demonstrated that implants placed into Bio-Oss grafted bone had the same degree of osseointegration as intact alveolar ridges and that the graft particles were separated from the implant surface by the vital mineralized bone. Bio-Oss graft particles are known to resorb and be replaced with bone slowly.
Based on a review of the literature,12-20 it appears that among biocompatible xenogeneic bone-substitute grafting materials with osteoconductive qualities, Bio-Oss is one of the leading products with a consistent performance.
Puros
Puros allograft is solvent-dehydrated, gamma-irradiated human cadaveric bone. Puros uses the Tutoplast® (Tutogen Medical, Inc, www.rtix.com) process of sterilization, which effectively prevents disease transmission of Creutzfeldt-Jacob Disease, hepatitis, and others. Mineralized and demineralized allografts have been successfully used alone or in composite grafts for many decades. Puros is a collagen matrix-preserved bone allograft that has been used effectively in a variety of dental applications, including periodontal osseous defects,21 socket grafting with subsequent placement of endosseous implants,22 and sinus grafting.23 Minichetti and colleagues22 showed a 97.6% implant success rate within the first year with mineralized human allograft (Puros) that previously was placed into extraction sites. In their study, Puros appeared clinically reliable to maintain bone volume and provide bone augmentation acceptable for implant placement.
Based on a review of the literature,21-23 it appears that among biocompatible allogeneic bone-substitute grafting materials with osteoconductive qualities, Puros allograft is one of the better products with dependable outcomes.
Comparisons of Bio-Oss and Puros
Comparisons of Bio-Oss and Puros can be found in few literature reports. Froum et al,24 in a blinded, randomized, controlled investigation, histomorphometrically evaluated the vital bone formed following 13 bilateral sinus grafts with two different materials: Puros and Bio-Oss. Bone cores were obtained and analyzed at 26 and 32 weeks. Authors concluded that significantly more bone was formed in the Puros sites. Histologically, both Puros and Bio-Oss particles were surrounded by new bone, osteoid, and osteoblasts. A higher average percentage of new vital bone was seen around Puros particles than around Bio-Oss particles. Noumbissi et al23 concluded from their study that all Puros grafts used for sinus augmentation fully resorbed and were replaced by newly formed bone faster and in greater quantities than in control (bovine) grafts. No differences in osseointegration or graft stability were noted 2 years after the study. Vance et al,25 in a randomized, controlled, blinded clinical study, compared ridge dimensions and histologic characteristics of ridges preserved with two different graft materials—allograft and bovine-derived xenograft—on 24 patients who required extraction and delayed implant placement. Trephine core biopsies 4 months after extraction demonstrated allograft mixed with an experimental putty carrier produced significantly more vital bone fill than did the use of a xenograft with no carrier material. Ridge width and height dimensions were preserved similarly with both graft materials.
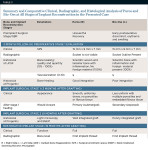
Table 4 details a comparison of the two bone graft materials, based on a review of the literature, the presented study, and the authors' experience with hundreds of cases of socket bone grafting with either Puros or Bio-Oss.
It appears that both grafting materials (Puros and Bio-Oss) have shown consistent, reliable, and comparable ridge preservation capabilities, especially in socket/alveolar grafting and subantral augmentation cases. It is also evident that Puros graft is being resorbed and replaced with vital and vascularized bone consistently faster and to a higher degree than Bio-Oss graft. Bio-Oss often is surrounded by fibrous tissue and takes longer to incorporate into the host bone.
At the same time, it seems that, if not functionally loaded with endosseous implants relatively soon (within 4 to 6 months), Puros-augmented alveolar bone tends to diminish in size and volume (resorb).14,16 Conversely, Bio-Oss graft tends to form a more stable and lasting bony foundation (due to its slow resorption) in augmented alveolar ridges and subantral sites with little or no resorptive potential in the future.12-14,18,19
Conclusion
The primary goal of many innovative bone grafting approaches is to preserve or improve the alveolar ridge anatomy, creating a foundation for osseointegrated implants to be restored successfully to normal function, esthetics, phonetics, and comfort. Success in ridge preservation (soft and hard tissue) is extremely important for optimizing implant function.
The presented case report analyzed and compared two bone graft materials used in clinical practice. It showed similar success of both Puros and Bio-Oss for socket preservation after tooth extraction. Biologically, it appears that Puros behaves more like a true bone graft, generating bone that is similar to the host bone in its density and vitality. Bio-Oss produces a more calcified bone and behaves more like bone graft substitute or bone filler. In spite of poor initial mineralization of the Bio-Oss sites tested 3 months after socket grafting (about 20%), a moderately good and equivalent degree of bone vascularization on both sides (scale 5) can suggest as good a potential for implant integration into the Bio-Oss grafted bone as into the Puros bone.
In cases when patients are committed to implant treatment after socket graft, both grafting materials (Puros and Bio-Oss) can be effective. When patients are not committed to implant treatment in the short term, the slowly resorbable Bio-Oss can provide more stable bone with relatively long maintenance of its volume.
Long-term prospective animal and human studies with clinical, radiographic, and histological assessments of different bone grafting materials and their mixtures used for alveolar bone augmentation should be able to help determine the best grafting solution for every jaw zone and every implant reconstructive situation aiming to achieve ideal functional bone stability and vitality, as well as implant success.
Acknowledgment
All surgeries were performed by Len Tolstunov, DDS; restorative treatment was performed by Diane Hiura, DDS. The authors greatly appreciate the help of Prof. Dr. Joost D. de Bruijn from The Netherlands and Dr. Richard C. K. Jordan from the Unites States (UCSF) for the histological examination and analysis of bone samples in preparation for this article.
References
1. Elian N, Cho SC, Froum S, et al. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;19(2):99-106.
2. Wang HL, Tsao YP. Mineralized bone allograft-plug socket augmentation: rationale and technique. Implant Dent. 2007;16(1):33-41.
3. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377-396.
4. Irinakis T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J Can Dent Assoc. 2006;72(10):917-922.
5. Otto M. Socket preservation. SADJ. 2006;61(8):366-368.
6. Bader H. Immediate extraction site grafting: materials and clinical objectives. Dent Today. 2005;24(7):86-89.
7. Wang HL, Kiyonobu K, Neiva RF. Socket augmentation: rationale and technique. Implant Dent. 2004;13(4):286-296.
8. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral and Maxillofac Implants. 2004;19(4);491-497.
9. Boyne P, Jones SD. Demonstration of the osseoinductive effect of bone morphogenetic protein within endosseous dental implants. Implant Dent. 2004;13(2):180-184.
10. Boyne PJ. Application of bone morphogenetic proteins in the treatment of clinical oral and maxillofacial osseous defects. J Bone Joint Surg Am. 2001;83-A(suppl 1, pt 2):S146-S150.
11. McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int Orthop. 2007;31(6):729-734.
12. Sclar A. Ridge preservation for optimal esthetics and function: the Bio-Col technique. Postgraduate Dent. 1999;6(1):3-11.
13. Sclar AG. Strategies for management of single-tooth extractions sites in aesthetic implant therapy. J Oral Maxillofac Surg. 2004;62(9 suppl 2):90-105.
14. Valentini P, Abensur D. Maxillary sinus floor elevation for implant placement with demineralized freeze-dried bone and bovine bone (Bio-Oss): a clinical study of 20 patients. Int J Periodontics Restorative Dent. 1997;17(3):232-241.
15. Warrer L, Gotfredsen K, Hjørting-Hansen E, Karring T. Guided tissue regeneration ensures osseointegration of dental implants placed into extraction sockets. An experimental study in monkeys. Clin Oral Implants Res. 1991;2(4):166-171.
16. Wetzel AC, Stich H, Caffese RG. Bone apposition onto oral implants in the sinus area filled with different grafting materials. A histological study in beagle dogs. Clin Oral Implants Res. 1995;6(3):155-163.
17. Zitzmann NU, Naef R, Schärer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration [published erratum appears in Int J Oral Maxillofac Implants. 1998;13(4):576]. Int J Oral Maxillofac Implants. 1997;12(6):844-852.
18. Artzi Z, Nemcovsky CE, Tal H. Efficacy of porous bovine bone mineral in various types of osseous deficiencies: clinical observations and literature review. Int J Periodontics Restorative Dent. 2001;21(4):395-405.
19. Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997;8(2):117-124.
20. Block MS, Sclar T, eds. Soft Tissue Esthetic Procedures for Teeth and Implants. Atlas of Oral and Maxillofacial Surgery Clinics. Philadelphia, PA: W.B. Saunders; 1999;7(2)39-59.
21. Vastardis S, Yukna RA. Evaluation of allogeneic bone graft substitute for treatment of periodontal osseous defects: 6-month clinical results. Compend Contin Educ Dent. 2006;27(1):38-44.
22. Minichetti JC, D'Amore JC, Hong AY. Three-year analysis of Tapered Screw-Vent implants placed into extraction sockets grafted with mineralized bone allograft. J Oral Implantol. 2005;31(6):283-293.
23. Noumbissi SS, Lozada JL, Boyne PJ, et al. Clinical, histologic, and histomorphometric evaluation of mineralized solvent-dehydrated bone allograft (Puros) in human maxillary sinus grafts. J Oral Implantol. 2005;31(4):171-179.
24. Froum SJ, Wallace SS, Elian N, et al. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006;26(6):543-551.
25. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
About the Authors
Len Tolstunov, DDS
Private Practice Limited to Oral and Maxillofacial Surgery
San Francisco, California
Assistant Clinical Professor
Department of Oral and Maxillofacial Surgery
University of the Pacific School of Dentistry
San Francisco, California
Jibin Chi, MD, MBA, MBI
Managing Director
Channel Innomatrix
The Netherlands