Mechanism of Action of a Desensitizing Fluoride
Anthony E. Winston, BSc; Andrew J. Charig, BS; and Stephen Thong, PhD
Abstract
It is generally accepted that the pain of dental hypersensitivity resulting from gum recession is from the movement of fluid within the exposed tubules of dentin, causing changes in pressure on the nerve within the pulpal cavity. One method of treating hypersensitivity is to occlude the tubules, preventing fluid movement. This article discusses the use of a dye penetration technique, which establishes this mechanism of action for a desensitizing fluoride toothpaste containing calcium and phosphate. Two groups of intact teeth were perfectly sealed with enamel paint. Windows 100-µ to 200-µ deep were opened on opposite sides of each tooth at the dentin-enamel junction and briefly etched using 20% polyacrylic acid. One batch of teeth was treated eight times for 30 mins each with a 1: 3 slurry of the desensitizing toothpaste and another set with a similar slurry prepared from a calcium- and phosphate-free control. A 0.85% aqueous solution of acid red fuchsin dye was applied to each window and allowed to dry. After a brief rinse, the teeth were sectioned across the windows. Almost no dye penetration was seen in teeth treated with the desensitizing toothpaste; however, extensive penetration through the dentin was visible in the control-treated teeth. The differences in dye penetration for the two sets of teeth were significant by both subjective (P < .001) and objective (P < .01) measures. Tubule occlusion because of calcium and phosphate ions from the desensitizing toothpaste accounts for its tooth desensitizing efficacy.
Dentin hypersensitivity is a common problem in which moderate-to-serious tooth discomfort is experienced because of mild nondamaging stimuli and is not associated with any significant tooth defect, disease, or pathology.1-5 The classic definition of dentine hypersensitivity characterizes the pain as short and sharp.1-4 However, sometimes the initial pain is severe and followed by a lingering dull ache.
The exposed portion of a healthy tooth is covered by an impenetrable hard-enamel mineral layer covering the softer inner dentin. The enamel layer ends just below the gum line so that the dentin below the cervical enamel junction is normally protected from exposure by the gum. However, in relatively early adulthood, the gums tend to recede, exposing the dentin. Initially, it is covered with a thin protective layer of cementum but rapidly becomes exposed when this layer is inevitably lost because of toothbrush abrasion or dissolution by acidic beverages and foods.1-4
Dentin is permeated with many tiny tubules providing passageways from the dentin surface to the pulpal cavity in which the nerves are housed.5 According to the generally accepted hydrodynamic theory, the pain of hypersensitivity is caused by pressure on the nerves, resulting from changes in fluid flow within the exposed dentinal tubules.1-8 Changes in fluid flow are caused by variations in external conditions around the tooth, such as osmotic, thermal, or tactile stimuli.
It should be noted that gingival recession is not the only cause of hypersensitivity. Access to the dentin can occur because of enamel loss or cracking resulting from attrition, abrasion, erosion, caries, or other trauma, which can also give rise to sensitivity.2,3 However, this more serious cause of pain and sensitivity is not generally amenable to treatment with a toothpaste but needs intervention by a dentist.
Two types of treatments are available for tooth hypersensitivity. Nerve activity and neural transmission can be arrested with suitable medications. Thus, for example, direct application of a solution or toothpaste containing potassium nitrate to the exposed dentin can depolarize the nerves and block neural transmission.4-6,8-13 This method of treatment is likely to be relatively short-lived because the effect of such medications will last only as long as some of the medication remains in place. Because hypersensitivity is often episodic in nature, this form of treatment may be adequate in some patients.4
A second method of treating dental hypersensitivity is to occlude the tubules, thereby blocking access to the nerves and stopping fluid flow within the tubules.2-6,8 Many effective tubule-occluding substances have been identified and successfully employed to relieve hypersensitivity. An advantage of this treatment is that it can potentially provide longer-term and possibly permanent hypersensitivity relief.
The fluoride toothpaste evaluated in this study is designed to occlude tubules with the same type of hard mineral of which tooth enamel is constituted. In previous research, the effectiveness of this toothpaste in occluding tubules was established using coronal dentin, and the nature of the occluding deposit was determined.14,15 In the absence of other toothpaste ingredients, the combination of calcium, phosphate, and fluoride components of the toothpaste was shown to occlude tubules and reduce fluid flow through coronal dentin in the standard dentin permeability test using Pashley cells.14 Furthermore, the comparability of the mechanism of action of this toothpaste to a professional desensitizer, which is known to desensitize teeth by depositing a layer of amorphous calcium phosphate (ACP) on dentin and blocking tubules, was demonstrated.15 In contrast, as expected, potassium nitrate solution, which does not lead to tubule blockage, did not inhibit fluid diffusion in this test. In addition, the fluoride toothpaste containing the same calcium and phosphate components but with other insoluble ingredients removed was more effective at inhibiting fluid flow through a Pashley cell than its corresponding calcium- and phosphate-free placebo. Thus, the evidence indicates that this dentifrice deposits ACP onto the surface of the dentin, covering the tubule openings, when the calcium and phosphate components combine in contact with saliva in the mouth. Subsequently, the ACP transforms into apatitic tooth mineral, forming a coherent layer, which is strongly bound to the dentin.
A clinical study has shown the toothpaste to reduce tooth hypersensitivity to a significantly greater degree than a conventional fluoride toothpaste used as a control.16 The reduction in hypersensitivity obtained from use of the dentifrice was maintained for at least 1 month after discontinuance of the toothpaste.16 These results, taken together, provide strong presumptive evidence that the effectiveness of the toothpaste in controlling hypersensitivity is from its ability to occlude dentinal tubules.
Ideally, to unequivocally prove the mechanism of action of this toothpaste, it would be desirable to demonstrate that the toothpaste reduces dentin permeability more effectively than a control toothpaste with a similar formulation except without the calcium and phosphate. However, the standard in vitro dentin permeability test, using coronal dentin in Pashley Cells, was unable to distinguish differences in permeability reduction due to the complete fluoride toothpaste formulation from the calcium- and phosphate-free control or a commercial desensitizing toothpaste, designed to depolarize the nerves using potassium ions.14 It was found that both the control and potassium nitrate-containing toothpastes left residues of insoluble ingredients from the dentifrices on the dentin. It appears that the presence of these insoluble ingredients was responsible for large reductions in dentin permeability that were achieved with these dentifrices. Pashley et al similarly found that, despite their supposed clinical ineffectiveness, insoluble ingredients such as toothpaste abrasives reduce dentin permeability.8,17 In many cases, they determined the reduction in dentin permeability as a result of treatment with a desensitizing toothpaste was indistinguishable from that due to conventional toothpastes. This inability could be from significant differences between real-life conditions and those present in the Pashley cell.
This current study discusses evaluation of the desensitizing toothpaste, using a different dentin permeability technique that employs dye penetration. The purpose was thus to unequivocally establish that the desensitizing effectiveness of the toothpaste is caused by tubule occlusion, resulting from the presence of the calcium and phosphate ingredients in the dentifrice formulation. In this study, the ability of the complete toothpaste formulation to prevent fluid flow through dentinal tubules at the cervical-enamel junction was compared to that of a calcium- and phosphate-free control toothpaste that otherwise contained the same ingredients as the complete toothpaste formulation.
Materials and Methods
Enamel Care® Sensitive Toothpaste (Church & Dwight Co Inc, https://www.churchdwight.com), with 6% calcium sulfate and 1.8% potassium phosphate, was compared with a control toothpaste that lacked calcium and phosphate. Both toothpastes also contained 1100-ppm fluoride and equal levels of baking soda, humectant, silica and organic thickeners, titanium dioxide, flavor, and sweetener.
After cleaning, two approximately balanced sets of nine intact teeth were dried briefly in a warm oven and completely sealed against any spurious dye penetration by twice immersing the teeth in enamel paint (Rust-Oleum®, Rust-Oleum Corp., https://www.rustoleum.com) and drying after each immersion. After further drying in a warm oven overnight, the teeth were carefully inspected for cracks or openings and found to be completely sealed.
Two windows were made on opposite sides of the teeth through the paint on each tooth at the dentin-enamel junction using a powered hand tool (Dremel®, https://www.dremel.com) with a 2.4-mm titanium carbide tip (No. 9904). The window extended about 1 mm to 2 mm up into the enamel area and a similar distance down into the dentin area; in the process, about 100 µ to 200 µ of tooth mineral was abraded away in the window area. Each exposed area was then etched for 15 sec using 20% polyacrylic acid (Good-Rite® K702, Noveon, Inc, https://www.lubrizol.com) and rinsed thoroughly with water.
Slurries of each toothpaste were prepared by mixing 7 g of toothpaste with 21 g of water. This dilution is typical of conditions found in the mouth during brushing with a toothpaste because of dilution with saliva. Each batch of teeth was treated eight times by total immersion in one of the freshly prepared toothpaste slurries for 30 min while being gently shaken. The teeth were briefly rinsed in water between treatments and at the end of the final treatment. At the completion of the treatments, the teeth were allowed to air dry.
Acid red fuchsin dye was applied as a 0.85% solution in water from a dropping pipette onto each window and left to penetrate and dry for 2 to 3 hours. The teeth were then briefly rinsed.
The teeth were sectioned vertically across the two windows using a Buelher IsoMet® (Buehler Ltd., https://www.buehler.com) saw with a diamond blade to provide 18 halves for each treatment. The cut faces were polished on Carbimet papers (Buehler Ltd) at, successively, 600 grit, 800 grit, and 1200 grit, and finally with a 50-nm diamond suspension on a polishing cloth.
Two methods of comparison were used to assess the effectiveness of the treatment in preventing diffusion of dyed solution into the tubules. In one, each tooth half was subjectively evaluated for dye penetration by two examiners and rated using a four-point scale: 0 = none; 1 = detectable; 3 = substantial; and 4 = extensive. The results from the two examiners were added. The significance of differences for the two sets of teeth was then determined using a chi-square test.
With the second method, the teeth were digitally photographed using a color camera and the photographs were scanned into Photoshop® (Adobe Systems Inc, https://www.adobe.com). Image analysis was then used to estimate the proportion of red within each tooth. The contrast between the dyed and nondyed areas was enhanced by adjusting the hue and saturation in Photoshop, and the colored area was outlined with the lasso tool. The counts of unlit green pixels were then recorded, and results for each set of teeth were averaged. A Student’s t-test was used to compare the results.
Results
After treatment but before application of the dye solution, there were signs of residue on both sets of teeth in the area of the exposed windows. On the product-treated teeth, the deposited material looked hard. However, on the placebo-treated teeth, the deposit appeared powdery. After application of the dye solution and rinsing, traces of dye were in or on the outside surface of all 18 teeth at the abraded areas.
Of the tooth halves treated with the test toothpaste, about three-fifths showed no signs of dye penetration, while the rest showed minimal amounts of dye. Conversely, 85% of the placebo-treated tooth halves had substantial or extensive dye permeation within the dentin, with the other 15% of the teeth showing detectable amounts of dye penetration. Close examination of the tooth halves showed that virtually all of the dye penetration within the placebo-treated teeth was through the dentin, presumably through the tubules; neither treatment group showed any signs of penetration through the apical foramina or any regions other than the specially abraded areas, confirming that the paint seal was effective.
Table 1 provides a summary of the subjective assessment of dye permeation within the dentin for the two sets of teeth. A chi-square test shows there is very significantly more dye penetration in the tooth halves treated with the placebo than the toothpaste containing calcium and phosphate (P < .001).
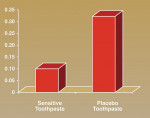
Figure 1 (View Figure) provides an objective comparison of the proportion of red pixels, corresponding to areas of dye penetration, within the dentin of each set of tooth halves. A Student’s t-test comparing the pixel counts shows the level of dye permeation within the placebo-treated tooth halves is significantly greater than within the halves treated with the toothpaste containing calcium and phosphate (P < .01)
Discussion
Previous studies have shown the ability of the fluoride toothpaste that delivers calcium and phosphate to occlude tubules in dentin by depositing ACP.14,15 However, the permeability test using coronal dentin was unable to distinguish the performance of this toothpaste from a placebo dentifrice and a potassium phosphate-containing desensitizing toothpaste, presumably because of the presence of insoluble ingredients such as titanium dioxide and silicas in the formulations.15
The inability of the dentin permeability test and other determinations of tubule occlusion to consistently differentiate between toothpaste formulations that are effective in vivo from ineffective ones has been noted by others.5,8,17-22 For example, Pashley et al obtained 20% to 50% reductions in dentinal flow rate by a single application of five placebo dentifrices.17 In those comparisons, the performance of the only marketed tubule-occluding desensitizing toothpaste (containing strontium chloride) was indistinguishable from its active-free control. One explanation may be provided by findings that fine particle layers on dentin, which have have been shown to produce a 90% reduction in hydrostatic-driven fluid flow, sometimes only result in a 50% reduction in flow because of an air blast.23,24 The residual evaporative-induced flow may still be sufficient to induce pain. Another explanation might be that the substrates used in the standard dentin permeability test are disks cut from coronal dentin. These, for many reasons, could perform appreciably differently from whole dentin in exposed roots.
The dye penetration technique in this study used a more realistic substrate: exposed dentin at the dentin-enamel junction. Furthermore, in this test, the flow into the dentin was induced by low-force capillary action rather than by the hydrostatic-driven pressure in the standard dentin permeability test. Given that the tiny amount of evaporative-induced flow resulting from an air blast is sufficient to induce pain in hypersensitive teeth, this test should be more representative of the pressure changes that can induce pain.
In Pashley cell comparisons, toothpaste treatments are applied by brushing onto the surface of the dentin. Insoluble particles from the dentifrice are forced into the tubules by gravity and brushing force. These forces may cause the toothpaste insolubles to be more strongly trapped in the tubules even though they are not actually bonded to the dentin. As a result, they significantly reduce dentin permeability. In this dye penetration test, the toothpastes were applied passively by immersion into toothpaste slurries. There were almost no forces pushing the insoluble toothpaste ingredients into the tubules. However, the active tubule-blocking ingredients can precipitate directly on the dentin and bond with it.
As a result, this technique was able to distinguish between the clinically proven effective hypersensitivity formulation and a placebo. It should be noted that visible deposits of insoluble materials were seen to adhere to the dentin because of treatment with the placebo toothpaste but these materials did not prevent fluid flow through the tubules, as demonstrated by dye penetration. In contrast, the fully formulated toothpaste containing calcium and phosphate was highly effective in sealing the root dentin tubules and in preventing fluid flow therein.
Conclusion
The Sensitive toothpaste delivering calcium and phosphate to the dentin surface is highly effective in occluding dentinal tubules and preventing fluid flow within root dentin and significantly more effective than the calcium- and phosphate-free placebo. The ability of this toothpaste to ameliorate hypersensitivity is because of its ability to occlude dentinal tubules.
Disclosure
This research was supported by the Church & Dwight Co, Inc. Mr. Winston is a former Church & Dwight employee and serves as a consultant for the company. Mr. Charig is a former employee. Dr. Thong is a current employee of Church & Dwight.
References
1. Addy M. Dentin hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367-375.
2. Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity—an enigma? A review of terminology, epidemiology, mechanisms, aetiology and management. Br Dent J. 1999;187(11)606-611.
3. Kielbassa AM. Dentine hypersensitivity: simple steps for everyday diagnosis and measurement. Int Dent J. 2002;52:394-396.
4. Draper C. Dentin hypersensitivity: understanding the causes and treatment strategies. Contemporary Oral Hygiene. 2008;6(4):11-19.
5. Gillam, DG, Mordan, NJ, Newman HN. The Dentin Disc surface: a plausible model for dentin physiology and dentin sensitivity evaluation. Adv Dent Res. 1997;11(4): 487-501.
6. Walters PA. Dentinal hypersensitivity: a review. J Contemp Dent Pract. 2005;6(2):1-10.
7. Brannstrom M. A hydrodynamic mechanism in the transmission of pain producing stimuli through dentin. In: Sensory Mechanism in Dentine. Anderson DJ, ed. Oxford, UK: Pergammon Press;1963:73-79.
8. Sena FJ. Dentinal permeability in assessing therapeutic agents. Dent Clin North Am. 1990;34(3): 475-490.
9. Silverman, Berman E, Hanna CB, et al. Assessing the efficacy of three dentifrices in the treatment of dentinal hypersensitivity. J Am Dent Assoc. 1996;127(2): 191-201.
10. Tarbet WJ, Buckner A, Stark MM, et al. The pulpal effects of brushing with 5 percent potassium nitrate paste used for desensitization. Oral Surg Oral Med Oral Pathol. 1981;51(6): 600-602.
11. Poulsen S, Errboe M, Hovgaard O, et al. Potassium nitrate for dentine hypersensitivity (review). The Cochrane Collaboration. 2004;(4): 1-11.
12. Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain. 2000;14(1):9-19.
13. Wara-aswapati N, Krongnawakul D, Jiraviboon D, et al. The effect of a new toothpaste containing potassium nitrate and triclosan on gingical health, plaque formation and dentine hypersensitivity. J Clin Periodontol. 2005;32(1): 53-58.
14. Charig A, Chapin C, Major E, et al. Mechanism of action of a desensitizing fluoride toothpaste containing calcium and phosphate ingredients in the treatment of dental hypersensitivity. Part I: in vitro SEM, EDX, profilometry and dentin permeability studies. Compend Contin Educ Dent. 2009;30(8). https://www.compendiumlive.com/article.php?id=3047. Accessed October 10, 2009.
15. Charig A, Thong S, Flores F, et al. Mechanism of action of a fluoride toothpastes delivering calcium and phosphate ingredients for the treatment of hypersensitivity. Part II: comparison with a professional treatment for tooth hypersensitivity. Compend Contin Educ Dent. 2009;30(9):622-633.
16. Ghassemi A, Hooper W, Winston A, et al. Clinical study to determine the effect of a baking soda toothpaste delivering calcium and phosphate ingredients on dentinal hypersensitivity. J Clin Dent. In press.
17. Pashley DH, O’Meara JA, Kepler EE, et al. Dentin permeability effects of desensitizing dentifrices in vitro. J Periodontol. 1984;55(9):522-525.
18. West NX, Addy M, Hughes J. Dentine hypersensitivity: the effects of brushing desensitizing toothpastes, their solid and liquid phases, and detergents on dentine and acrylic: studies in vitro. J Oral Rehabil. 1998;25:885-895.
19. West NX, Hughes JA, Addy M. Dentine hypersensitivity: the effects of brushing toothpaste on etched and unetched dentine in vitro. J Oral Rehabil. 2002;29(2):167-174.
20. Addy M, Mostafa P. Dentine hypersensitivity. II. Effects produced by the uptake in vitro of toothpastes onto dentine. J Oral Rehabil. 1989;16(1):35-48.
21. Addy M, Urquhart E. Dentin hypersensitivity: its prevalence, aetiology and clinical management. Dent Update. 1992;19(10):407-412.
22. Absi EG, Addy M, Adams D. Dentine hypersensitivity: uptake of toothpastes onto dentine and effects of brushing washing and with dietary acid—SEM in vitro study. J Oral Rehabil. 1995;22(3):175-182.
23. Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35(4):300-315.
24. Pashley DH, Matthews WG, Zhang Y, et al. Fluid shifts across human dentine in vitro in response to hydrodynamic stimuli. Arch Oral Biol. 1996;41(11): 1065-1072.
About the Authors
Anthony E. Winston, BSc
President, R&D for Hire, LLC
East Brunswick, New Jersey
Andrew J. Charig, BS
Senior Chemist (retired)
Church & Dwight Co, Inc
Princeton, New Jersey
Stephen Thong, PhD
Director
Global Oral Care R&D for Oral Care Products
Church & Dwight Co, Inc
Princeton, New Jersey