Tissue-Specific, Direct-Application Class II Tooth Repair: A Case Report
Theodore P. Croll, DDS; Richard R. Cavanaugh, DDS
When orthopedic surgeons repair or replace osseous structures in human joints, they strive to use materials that have form and function to replicate what was lost. The bioengineering concept of “biomimesis” means mimicking natural biologic essence,1 and that is an important goal of healers.
Dentists also toil with biomimetic principles. Today, more than ever, dental clinicians and oral and maxillofacial surgery specialists have the ability to repair, replace, and restructure facial bones, dentitions, and individual teeth in such ways that biologic form, function, and appearance can be improved substantially.
Dentists should consider the concept of biomimesis when repairing individual teeth. Nowadays, certain types of precision-fabricated full-crown restorations are cemented into place with resin-modified glass-ionomer luting cements. Besides their hydrophilic nature, biocompatibility, chemical bond to dentin, fluoride ion release (and uptake by adjacent tooth structure), these cements have sufficient physical strengths and insolubility to withstand the harsh intraoral environment and the impact forces germane to occlusion and mastication. In short, resin-modified glass-ionomer luting cements serve as a biocompatible adhesively bonded extension of the remaining dentin. In the same way, the overlying artificial crown serves to replace the remaining coronal form.
Likewise, when a dentist directly repairs a tooth infected with dental caries or having structural deficit (malformation, wear, fracture, erosion) using directly applied adhesively bonded restorative materials, consideration should be given to separate, yet combined, “tissue-specific” restoration. It makes sense that if one restorative material is available that closely mimics dentin and another exists that closely replicates enamel, they should be used in concert to benefit from the advantages of each, and their synergy. A paramount advantage of stratifying these materials that makes for an ideal restorative model is that resin-modified glass-ionomer cement and resin-based composite combine chemically during the process, analogous to enamel and dentin biologic fusion at the dentino-enamel junction.
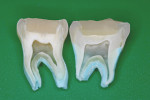
Mechanical interlocking retention form and virtual “entombment” of the dentin replacement base beneath the bonded resin-based composite mass—protecting the glass-ionomer material from virtually all influences that could affect its bond to tooth structure or challenge its physical strengths—additionally make stratification of materials advisable2-11 (Figure 1). Other advantages of tissue-specific restorative layering are reviewed in the discussion section.
Case Example
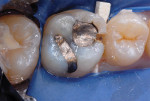
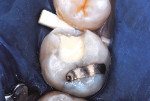
The procedure for tissue-specific, direct-application adhesive restoration of teeth is exemplified in the following technique description. A 29-year-old woman had fractured the mesial marginal ridge of a maxillary permanent first molar. The mesial fossa of the occlusal surface and occluso-lingual groove had been repaired years earlier with silver amalgam material (Figure 2). New caries infection was evident along with the damage from fracture. The tooth was repaired anew, using a resin-modified glass-ionomer base overlaid with resin-based composite.
Technique
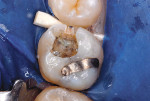
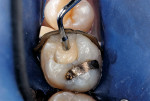
After appropriate local anesthetic injections, the tooth was isolated with a rubber dam, using the “slit-dam” method.12 First, attention was paid to the mesial section of the tooth. The silver amalgam material and fractured mesio-buccal enamel segment were removed with a water-cooled high-speed diamond bur and high-power evacuation (Figure 3). Deep penetration of caries infection was revealed. The carious substance was debrided with large slow-speed round burs. A small portion of hard-setting calcium hydroxide paste (Dycal®, DENTSPLY Caulk, www.caulk.com) was placed in the deepest portion of the preparation.
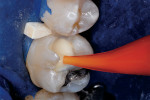
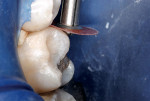
Then, resin-modified glass-ionomer liner (Vitrebond™ Plus, 3M ESPE, www.3MESPE.com) was blended, placed in a narrow lumen AccuDose® syringe tip (Centrix Inc, www.centrixdental.com), injected (Figure 4), and spread with an explorer tip to cover all exposed dentin. The liner was hardened with a 16-sec exposure to the visible light beam (1,200 mW/cm2). Next, the dentin-replacement liner/base was shaped with slow-speed carbide burs to replicate the dentin that had been lost to infection and preparation (Figure 5). Using a coarse small round diamond bur, the cavosurface margins and peripheral enamel were roughened. A custom-contoured 0.0015-in (0.0381-mm) stainless steel matrix strip then was placed and wedged firmly into place. Self-etching bonding agent (Adper™ Prompt™ L-Pop™, 3M ESPE) was applied, agitated for 30 sec, and light-polymerized. Four separate portions of resin-based composite (Filtek™ Supreme Plus, 3M ESPE), each light-cured after placement, were then injected and compressed into place with condensers and a large burnisher, in the usual manner (Figure 6 and Figure 7). Complete light polymerization of the final filled resin portion followed.
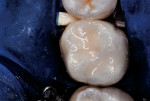
The overbuilt resin-based composite bulk then was sculpted into proper anatomic contours and occlusal form, using slow-speed diamond burs and aluminum-oxide abrasive discs of varying grits (Figure 8 and Figure 9). The resin surface and peripheral enamel then were painted with self-etching bonding agent. After 15 sec, a clear resin sealant material (Delton®, DENTSPLY Professional, www.dentsply.com) was applied and light-polymerized (Figure 10). The rubber dam was removed. Occlusal contacts were evaluated using articulating paper and adjustments made with round diamond burs at slow speed. Two years after treatment, the mesio-occlusal and occlusolingual restorations had surface wear-and-tear and slight marginal separations typically seen years after stress-bearing resin-based composite tooth repair (Figure 11). At that time, the restored surfaces were trimmed, smoothed, and resealed using a self-etching bonding agent and clear resin sealant, according to the method of Croll and Donly13 (Figure 12). A bitewing radiograph taken at 26 months after treatment confirmed proper form and contact of the repaired tooth (Figure 13).
Discussion
Ruiz and Mitra14 offered a detailed, research-based review about the use of cavity liners under posterior resin-based composite restorations. They cited the following advantages to use of a resin-modified glass-ionomer liner, such as Vitrebond Plus or Fuji LINING™ LC (GC America Inc, www.gcamerica.com):
- A liner serves as a “buffer” to counteract the contraction stresses of resin-based composite polymerization shrinkage. Such forces open gaps at the resin-tooth structure interface.
- Liners decrease cusp deformation, also associated with resin polymerization shrinkage during the light-polymerization process.
- They greatly decrease postoperative tooth sensitivity. This advantage of glass-ionomer dentin replacement is particularly important. Postoperative tooth sensitivity after resin-based composite tooth repair without glass-ionomer dentin replacement is exasperating for patients, parents, and dentists.15
- They wet the dentinal surfaces efficiently and, by chemical bonding that does not hydrolyze over time, adhere to tooth structure intimately.
- They have a coefficient of thermal expansion similar to that of tooth structure, which means temperature alterations influencing dimensional changes in the tooth will affect the resin-modified glass-ionomer material similarly. Such compatibility decreases potential for marginal opening and microleakage.
- Resin-modified glass-ionomer liners release fluoride ions for take-up by enamel and dentin, rendering those tissues less soluble to acid challenge. In addition, there is an antimicrobial effect because of the fluoride component of the calcium-aluminum-fluorosilicate glass filler.
An additional advantage of a resin-modified glass-ionomer base is seen when a carious marginal defect occurs in a previously repaired tooth. A dentist can easily re-repair a tooth that has such a defect, using new bonded material.16 However, that dentist cannot be certain of the extent of underlying caries involvement if resin-based composite is bonded directly to dentin. Usually, simple repair is not possible; the entire resin mass must be removed for accurate diagnostic assessment. A fluoride-containing, hydrophilic, chemically bonded resin-modified glass-ionomer base would inhibit the spread of caries infection. Although that assumption seems logical and appropriate, the ability of a dentin replacement resin-modified glass-ionomer liner/base to inhibit internal caries progression needs to be verified with laboratory and in vivo research.
In the future, stratification of glass-ionomer cements and resin-based composites may not be needed. Materials scientists are closing the gap between the physical properties of the respective adhesive restorative groups. Resin-modified glass-ionomer restorative cements have been shown to be durable and reliable, without resin-based composite stratification, for repair of primary teeth, usually until exfoliation.17,18 In 2007 a “nano-ionomer” was introduced commercially. Ketac™ Nano (3M ESPE) is a resin-modifed glass ionomer with improved physical characteristics, perhaps portending an eventual single repair material for simultaneous dentin and enamel replacement, which will last for the long term.19,20
Conclusion
Many articles in the scientific and commercial dental literature in the past decade have documented methods of repairing permanent posterior teeth, using resin-based com-posite directly bonded to dentin and enamel. Fewer articles have emphasized the history and advantages of repairing such teeth, using “tissue specific” materials such as reported here. This case report is offered to document an example of biomimetic direct application Class II tooth restoration, so that dentists can consider the implications of another option besides direct bonding of resin-based composite to dentin. When one reviews the above advantages of resin-modified glass-ionomer dentin replacement and considers that the only disadvantage might be the time it takes to place the liner or base prior to resin-based composite enamel restoration, tissue-specific tooth repair is justifiable and rational. The authors believe until a single tooth repair material is available for permanent teeth that replicates all favorable characteristics of both dentin and enamel, separate, yet combined, dentin and enamel replacement as described here should be considered the standard of care.
References
1. Bugliarello G. Biomimesis: the road less traveled. The Bridge. 1997;27(3): 2-3.
2. McLean JW, Wilson AD. The clinical development of the glass-ionomer cement. II. Some clinical applications. Aust Dent J. 1977;22(2):120-127.
3. McLean JW, Powis DR, Prosser HJ, et al. The use of glass-ionomer cements in bonding composite resins to dentine. Br Dent J. 1985;158(11): 410-414.
4. Wilson AD, McLean JW. Laminate restorations. In: Glass-Ionomer Cement. Chicago, IL: Quintessence Publishing Co; 1988:159-178.
5. Mount GJ. Clinical requirements for a successful “sandwich”-dentine to glass-ionomer cement to composite resin. Aust Dent J. 1989;34(3):259-265.
6. Croll TP. Replacement of defective Class I amalgam restorations with stratified glass ionomer-composite resin materials. Quintessence Int. 1989;20: 711-716.
7. Croll TP. Class I composite resin restoration. J Esthet Dent. 1992;4(5): 148-153.
8. Davidson CL. Glass-ionomer bases under posterior composites. J Esthet Dent. 1994;6(4):223-224.
9. Leinfelder KF. Changing restorative traditions: the use of bases and liners. J Am Dent Assoc. 1994;125(1):65-67.
10. Ferrari M. Use of glass-ionomers as bondings, linings, or bases. In: Davidson CL, Mjor IA, eds. Advances in Glass-Ionomer Cements. Chicago, IL: Quintessence Publishing Co; 1999:137-148.
11. Croll TP, Cavanaugh RR. Posterior resin-based composite restorations: a second opinion. J Esthet Restor Dent. 2002;14(5):303-312.
12. Croll TP. Alternative methods for use of the rubber dam. Quintessence Int. 1985;16(6):387-339.
13. Croll TP, Donly KJ. Resin-based composite margin repair. Inside Dentistry. 2008;4(6):92-93.
14. Ruiz JL, Mitra S. Using cavity liners with direct posterior composite restorations. Compend Contin Educ Dent. 2006;27(6):347-351.
15. Post-operative tooth sensitivity, clinician’s observations. CRA Newsletter. 1999;23(11):1-3.
16. Croll TP. Repair of Class I resin-composite restoration. ASDC J Dent Child. 1997;64(1):22-27.
17. Croll TP, Helpin ML, Donly KJ. Vitremer restorative cement for children: three clinicians’ observations in three pediatric dental practices. ASDC J Dent Child. 2000;67(6):391-339.
18. Croll TP, Bar-Zion Y, Segura A, et al. Clinical performance of resin-modified glass ionomer cement restorations in primary teeth. J Am Dent Assoc. 2001;132(8):1110-1116.
19. Croll TP, Nicholson JW. Glass-ionomer cements: history and current status. Inside Dentistry. 2008;4(3):76-84.
20. Croll TP, Berg JH. Nano-ionomer restorative cement: observations after 2 years of use. Inside Dentistry. 2009;5(1):60-67.
About the Authors
Theodore P. Croll, DDS
Private Practice
Pediatric Dentistry
Doylestown, Pennsylvania
Affiliate Professor
Department of Pediatric Dentistry
University of Washington School of Dentistry
Seattle, Washington
Adjunct Professor
Pediatric Dentistry
University of Texas Health Science Center at San Antonio Dental School
San Antonio, Texas
Richard R. Cavanaugh, DDS
Private Practice
Fixed and Implant Prosthodontics
Doylestown, Pennsylvania